Catalytically efficient Ni-NiOx-Y2O3 interface for medium temperature water-gas shift reaction
- PMID: 35508459
- PMCID: PMC9068818
- DOI: 10.1038/s41467-022-30138-5
Catalytically efficient Ni-NiOx-Y2O3 interface for medium temperature water-gas shift reaction
Abstract
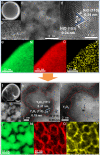
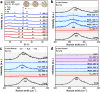
The metal-support interfaces between metals and oxide supports have long been studied in catalytic applications, thanks to their significance in structural stability and efficient catalytic activity. The metal-rare earth oxide interface is particularly interesting because these early transition cations have high electrophilicity, and therefore good binding strength with Lewis basic molecules, such as H2O. Based on this feature, here we design a highly efficient composite Ni-Y2O3 catalyst, which forms abundant active Ni-NiOx-Y2O3 interfaces under the water-gas shift (WGS) reaction condition, achieving 140.6 μmolCO gcat-1 s-1 rate at 300 °C, which is the highest activity for Ni-based catalysts. A combination of theory and ex/in situ experimental study suggests that Y2O3 helps H2O dissociation at the Ni-NiOx-Y2O3 interfaces, promoting this rate limiting step in the WGS reaction. Construction of such new interfacial structure for molecules activation holds great promise in many catalytic systems.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Tauster SJ, Fung SC, Garten RL. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978;100:170–175. doi: 10.1021/ja00469a029. - DOI
-
- Wang H, et al. Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nat. Catal. 2021;4:418–424. doi: 10.1038/s41929-021-00611-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

