Genome and transcriptome mechanisms driving cephalopod evolution
- PMID: 35508532
- PMCID: PMC9068888
- DOI: 10.1038/s41467-022-29748-w
Genome and transcriptome mechanisms driving cephalopod evolution
Abstract
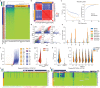
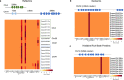
Cephalopods are known for their large nervous systems, complex behaviors and morphological innovations. To investigate the genomic underpinnings of these features, we assembled the chromosomes of the Boston market squid, Doryteuthis (Loligo) pealeii, and the California two-spot octopus, Octopus bimaculoides, and compared them with those of the Hawaiian bobtail squid, Euprymna scolopes. The genomes of the soft-bodied (coleoid) cephalopods are highly rearranged relative to other extant molluscs, indicating an intense, early burst of genome restructuring. The coleoid genomes feature multi-megabase, tandem arrays of genes associated with brain development and cephalopod-specific innovations. We find that a known coleoid hallmark, extensive A-to-I mRNA editing, displays two fundamentally distinct patterns: one exclusive to the nervous system and concentrated in genic sequences, the other widespread and directed toward repetitive elements. We conclude that coleoid novelty is mediated in part by substantial genome reorganization, gene family expansion, and tissue-dependent mRNA editing.
© 2022. The Author(s).
Conflict of interest statement
D.S.R. is a member of the Scientific Advisory Board of, and a minor shareholder in, Dovetail Genomics LLC, which provides as a service the high-throughput chromatin conformation capture (Hi-C) technology used in this study. The remaining authors declare no competing interests.
Figures






References
-
- Wang, Z. Y. & Ragsdale, C. W. Cephalopod nervous system organization. in Oxford Research Encyclopedia of Neuroscience (Oxford University Press, 2019).

