A comprehensive atlas of Aggrecan, Versican, Neurocan and Phosphacan expression across time in wildtype retina and in retinal degeneration
- PMID: 35508614
- PMCID: PMC9068689
- DOI: 10.1038/s41598-022-11204-w
A comprehensive atlas of Aggrecan, Versican, Neurocan and Phosphacan expression across time in wildtype retina and in retinal degeneration
Abstract
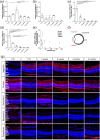
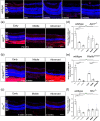
As photoreceptor cells die during retinal degeneration, the surrounding microenvironment undergoes significant changes that are increasingly recognized to play a prominent role in determining the efficacy of therapeutic interventions. Chondroitin Sulphate Proteoglycans (CSPGs) are a major component of the extracellular matrix that have been shown to inhibit neuronal regrowth and regeneration in the brain and spinal cord, but comparatively little is known about their expression in retinal degeneration. Here we provide a comprehensive atlas of the expression patterns of four individual CSPGs in three models of inherited retinal degeneration and wildtype mice. In wildtype mice, Aggrecan presented a biphasic expression, while Neurocan and Phosphacan expression declined dramatically with time and Versican expression remained broadly constant. In degeneration, Aggrecan expression increased markedly in Aipl1-/- and Pde6brd1/rd1, while Versican showed regional increases in the periphery of Rho-/- mice. Conversely, Neurocan and Phosphacan broadly decrease with time in all models. Our data reveal significant heterogeneity in the expression of individual CSPGs. Moreover, there are striking differences in the expression patterns of specific CSPGs in the diseased retina, compared with those reported following injury elsewhere in the CNS. Better understanding of the distinct distributions of individual CSPGs will contribute to creating more permissive microenvironments for neuro-regeneration and repair.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

