Liver-directed lentiviral gene therapy corrects hemophilia A mice and achieves normal-range factor VIII activity in non-human primates
- PMID: 35508619
- PMCID: PMC9068791
- DOI: 10.1038/s41467-022-30102-3
Liver-directed lentiviral gene therapy corrects hemophilia A mice and achieves normal-range factor VIII activity in non-human primates
Abstract
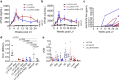
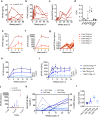
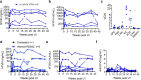
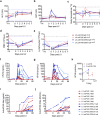
Liver gene therapy with adeno-associated viral (AAV) vectors delivering clotting factor transgenes into hepatocytes has shown multiyear therapeutic benefit in adults with hemophilia. However, the mostly episomal nature of AAV vectors challenges their application to young pediatric patients. We developed lentiviral vectors, which integrate in the host cell genome, that achieve efficient liver gene transfer in mice, dogs and non-human primates, by intravenous delivery. Here we first compare engineered coagulation factor VIII transgenes and show that codon-usage optimization improved expression 10-20-fold in hemophilia A mice and that inclusion of an unstructured XTEN peptide, known to increase the half-life of the payload protein, provided an additional >10-fold increase in overall factor VIII output in mice and non-human primates. Stable nearly life-long normal and above-normal factor VIII activity was achieved in hemophilia A mouse models. Overall, we show long-term factor VIII activity and restoration of hemostasis, by lentiviral gene therapy to hemophilia A mice and normal-range factor VIII activity in non-human primate, paving the way for potential clinical application.
© 2022. The Author(s).
Conflict of interest statement
L.N., A.C., A.A., M.M., T.L., S.P.W. are inventors on patent applications submitted by Foundation Telethon and San Raffaele Scientific Institute or Bioverativ/Sanofi on LV technology related to the work presented in this manuscript (WO2019/152692; WO2016009326). FT and SRSI, through SR-Tiget, have established a research collaboration on liver-directed lentiviral gene therapy of hemophilia with Bioverativ/Sanofi. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

