Improving clinical trials using Bayesian adaptive designs: a breast cancer example
- PMID: 35508968
- PMCID: PMC9066830
- DOI: 10.1186/s12874-022-01603-y
Improving clinical trials using Bayesian adaptive designs: a breast cancer example
Abstract
Background: To perform virtual re-executions of a breast cancer clinical trial with a time-to-event outcome to demonstrate what would have happened if the trial had used various Bayesian adaptive designs instead.
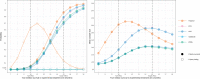
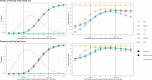
Methods: We aimed to retrospectively "re-execute" a randomised controlled trial that compared two chemotherapy regimens for women with metastatic breast cancer (ANZ 9311) using Bayesian adaptive designs. We used computer simulations to estimate the power and sample sizes of a large number of different candidate designs and shortlisted designs with the either highest power or the lowest average sample size. Using the real-world data, we explored what would have happened had ANZ 9311 been conducted using these shortlisted designs.
Results: We shortlisted ten adaptive designs that had higher power, lower average sample size, and a lower false positive rate, compared to the original trial design. Adaptive designs that prioritised small sample size reduced the average sample size by up to 37% when there was no clinical effect and by up to 17% at the target clinical effect. Adaptive designs that prioritised high power increased power by up to 5.9 percentage points without a corresponding increase in type I error. The performance of the adaptive designs when applied to the real-world ANZ 9311 data was consistent with the simulations.
Conclusion: The shortlisted Bayesian adaptive designs improved power or lowered the average sample size substantially. When designing new oncology trials, researchers should consider whether a Bayesian adaptive design may be beneficial.
Keywords: Bayesian adaptive trial; Predictive probability of success; Time-to-event.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

