Effects of conditioned medium obtained from human adipose-derived stem cells on skin inflammation
- PMID: 35509265
- PMCID: PMC9034017
- DOI: 10.1016/j.reth.2022.03.009
Effects of conditioned medium obtained from human adipose-derived stem cells on skin inflammation
Abstract
Introduction: Cell therapy using adipose-derived mesenchymal stem cells (ASCs) is a promising avenue of regenerative medicine for the treatment of various diseases. It has been considered that ASCs exert their therapeutic effects through the secretion of multiple factors that are critical for tissue remodeling or the suppression of inflammation. Recently, conditioned medium (CM) from ASCs that contains a complex of secreted factors has received attention as a cost-effective alternative to cell therapy.
Methods: We investigated the effects of CM obtained from ASCs (ASCs-CM) using human dermal fibroblasts (hDFs) and human epidermal keratinocytes with or without interleukin (IL)-1β and examined mRNA levels of marker genes. We also examined alterations in cell proliferation and morphology of hDFs following treatment with ASCs-CM. We further investigated the effects of ASCs-CM treatment on prevention of skin inflammation using a mouse model.
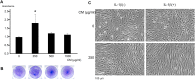
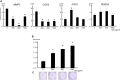
Results: In hDFs and human epidermal keratinocytes, the ASCs-CM treatment suppressed pro-inflammatory factors and enhanced regenerative and remodeling factors with or without interleukin (IL)-1β exposure. The ASCs-CM treatment also enhanced cell proliferation of hDFs and prevented morphological changes in response to IL-1β exposure. Furthermore, in a mouse model of skin inflammation, treatment with ASCs-CM reduced the inflammatory reactions, including redness and thickness.
Conclusions: CM from ASCs may represent a potential alternative to ASC therapy for the treatment of inflammatory skin conditions.
Keywords: ASC therapy; Adipose-derived mesenchymal stem cells (ASCs); Conditioned medium (CM); Human dermal fibroblasts (hDFs); Interleukin (IL)-1β; Skin inflammation.
© 2022 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Effects of conditioned medium from LL-37 treated adipose stem cells on human fibroblast migration.Exp Ther Med. 2017 Jul;14(1):723-729. doi: 10.3892/etm.2017.4558. Epub 2017 Jun 7. Exp Ther Med. 2017. PMID: 28672990 Free PMC article.
-
Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling.Stem Cells Transl Med. 2016 Jun;5(6):816-25. doi: 10.5966/sctm.2015-0191. Epub 2016 Apr 21. Stem Cells Transl Med. 2016. PMID: 27102647 Free PMC article.
-
IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology.Front Immunol. 2019 May 27;10:1075. doi: 10.3389/fimmu.2019.01075. eCollection 2019. Front Immunol. 2019. PMID: 31191517 Free PMC article.
-
Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints.Expert Opin Biol Ther. 2010 Apr;10(4):495-503. doi: 10.1517/14712591003610598. Expert Opin Biol Ther. 2010. PMID: 20218919 Review.
-
Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential.Expert Opin Biol Ther. 2009 Dec;9(12):1499-508. doi: 10.1517/14712590903307362. Expert Opin Biol Ther. 2009. PMID: 19780713 Review.
Cited by
-
Adipose-Derived Stromal Cells for Chronic Wounds: Scientific Evidence and Roadmap Toward Clinical Practice.Stem Cells Transl Med. 2023 Jan 30;12(1):17-25. doi: 10.1093/stcltm/szac081. Stem Cells Transl Med. 2023. PMID: 36571216 Free PMC article. Review.
-
The potential of Spirulina maxima extract-treated conditioned medium from adipose-derived mesenchymal stem cells for hair growth promoting effect on human dermal papilla cells.Regen Ther. 2025 Mar 26;29:184-191. doi: 10.1016/j.reth.2025.03.013. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40225048 Free PMC article.
-
The tardigrade-derived mitochondrial abundant heat soluble protein improves adipose-derived stem cell survival against representative stressors.Sci Rep. 2024 May 23;14(1):11834. doi: 10.1038/s41598-024-62693-w. Sci Rep. 2024. PMID: 38783150 Free PMC article.
-
Neural stem cell-derived extracellular vesicles alleviate inflammatory responses in a mouse model of atopic dermatitis.Stem Cells. 2025 Jul 21;43(8):sxaf034. doi: 10.1093/stmcls/sxaf034. Stem Cells. 2025. PMID: 40442899 Free PMC article.
-
Integration of multi-omics and single-cell transcriptome reveals mitochondrial outer membrane protein-2 (MTX-2) as a prognostic biomarker and characterizes ubiquinone metabolism in lung adenocarcinoma.J Cancer. 2025 Apr 13;16(7):2401-2420. doi: 10.7150/jca.106902. eCollection 2025. J Cancer. 2025. PMID: 40302794 Free PMC article.
References
-
- Jevotovsky D.S., Alfonso A.R., Einhorn T.A., Chiu E.S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage. 2018;26(6):711–729. - PubMed
-
- Golchin A., Farahany T.Z., Khojasteh A., Soleimanifar F., Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: an update and concise review. Curr Stem Cell Res Ther. 2019;14(1):22–33. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

