Antibody and T Cell Immune Responses to SARS-CoV-2 Peptides in COVID-19 Convalescent Patients
- PMID: 35509311
- PMCID: PMC9058163
- DOI: 10.3389/fmicb.2022.842232
Antibody and T Cell Immune Responses to SARS-CoV-2 Peptides in COVID-19 Convalescent Patients
Abstract
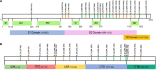
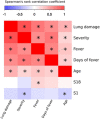
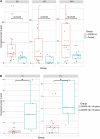
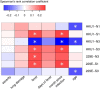
Identifying immunogenic targets of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is critical to advance diagnostic and disease control strategies. We analyzed humoral (ELISA) and T-cell (ELISpot) immune responses to spike (S) and nucleocapsid (N) SARS-CoV-2 proteins as well as to human endemic coronavirus (eCoV) peptides in serum from convalescent coronavirus disease 2019 (COVID-19) patients from Tatarstan, Russia. We identified multiple SARS-CoV-2 peptides that were reactive with serum antibodies and T cells from convalescent COVID-19. In addition, age and gender associated differences in the reactivity to S and N protein peptides were identified. Moreover, several SARS-CoV-2 peptides tested negatively correlated with disease severity and lung damage. Cross-reactivity to eCoV peptides was analyzed and found to be lower in COVID-19 compared to controls. In this study, we demonstrate the changing pattern of immunogenic peptide reactivity in COVID-19 serum based on age, gender and previous exposure to eCoVs. These data highlight how humoral immune responses and cytotoxic T cell responses to some of these peptides could contribute to SARS-CoV-2 pathogenesis.
Keywords: COVID-19; SARS-CoV-2; antibody humoral immune response; peptides; spike protein.
Copyright © 2022 Garanina, Hamza, Stott-Marshall, Martynova, Markelova, Davidyuk, Shakirova, Kaushal, Baranwal, Khaertynova, Rizvanov, Foster and Khaiboullina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Reactive T Cells in Convalescent COVID-19 Patients With Negative SARS-CoV-2 Antibody Serology.Front Immunol. 2021 Jul 12;12:687449. doi: 10.3389/fimmu.2021.687449. eCollection 2021. Front Immunol. 2021. PMID: 34322120 Free PMC article.
-
SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity.Am J Trop Med Hyg. 2021 Jun 17;105(2):395-400. doi: 10.4269/ajtmh.20-1594. Am J Trop Med Hyg. 2021. PMID: 34143752 Free PMC article.
-
The Potential of Developing Pan-Coronaviral Antibodies to Spike Peptides in Convalescent COVID-19 Patients.Arch Immunol Ther Exp (Warsz). 2021 Mar 6;69(1):5. doi: 10.1007/s00005-021-00607-8. Arch Immunol Ther Exp (Warsz). 2021. PMID: 33677719 Free PMC article.
-
SARS-CoV-2 recombinant proteins stimulate distinct cellular and humoral immune response profiles in samples from COVID-19 convalescent patients.Clinics (Sao Paulo). 2021 Dec 6;76:e3548. doi: 10.6061/clinics/2021/e3548. eCollection 2021. Clinics (Sao Paulo). 2021. PMID: 34878034 Free PMC article.
-
Diverse Humoral Immune Responses in Younger and Older Adult COVID-19 Patients.mBio. 2021 Jun 29;12(3):e0122921. doi: 10.1128/mBio.01229-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182775 Free PMC article.
Cited by
-
Neutralizing Antibodies in COVID-19 Serum from Tatarstan, Russia.Int J Mol Sci. 2023 Jun 15;24(12):10181. doi: 10.3390/ijms241210181. Int J Mol Sci. 2023. PMID: 37373331 Free PMC article.
-
Long-term humoral and cellular immunity after primary SARS-CoV-2 infection: a 20-month longitudinal study.BMC Immunol. 2023 Nov 16;24(1):45. doi: 10.1186/s12865-023-00583-y. BMC Immunol. 2023. PMID: 37974069 Free PMC article.
References
-
- Aguilar-Bretones M., Westerhuis B. M., Raadsen M. P., de Bruin E., Chandler F. D., Okba N. M., et al. (2021). Seasonal coronavirus–specific B cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J. Clin. Invest. 131:e150613. 10.1172/JCI150613 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

