Exogenous Production of N-acetylmuramyl-L Alanine Amidase (LysM2) from Siphoviridae Phage Affecting Anti-Gram-Negative Bacteria: Evaluation of Its Structure and Function
- PMID: 35509364
- PMCID: PMC9017466
- DOI: 10.18502/ajmb.v14i1.8169
Exogenous Production of N-acetylmuramyl-L Alanine Amidase (LysM2) from Siphoviridae Phage Affecting Anti-Gram-Negative Bacteria: Evaluation of Its Structure and Function
Abstract
Background: To obtain endolysin with impact(s) on gram-negative bacteria as well as gram-positive bacteria, N-acetylmuramyl L-alanine-amidase (MurNAc-LAA) from a Bacillus subtilis-hosted Siphoviridae phage (SPP1 phage, Subtilis Phage Pavia 1) was exogenously expressed in Escherichia coli (E. coli).
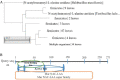
Methods: The sequences of MurNAc-LAA genes encoding peptidoglycan hydrolases were obtained from the Virus-Host database. The sequence of MurNAc-LAA was optimized by GenScript software to generate MurNAc-LAA-MMI (LysM2) for optimal expression in E. coli. Furthermore, the structure and function of LysM2 was evaluated in silico. The optimized gene was synthesized, subcloned in the pET28a, and expressed in E. coli BL21(DE3). The antibacterial effects of the protein on the peptidoglycan substrates were studied.
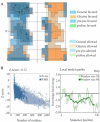
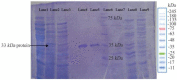
Results: LysM2, on 816 bp gene encoding a 33 kDa protein was confirmed as specific SPP1 phage enzyme. The enzyme is composed of 271 amino acids, with a half-life of 10 hr in E. coli. In silico analyses showed 34.2% alpha-helix in the secondary structure, hydrophobic N-terminal, and lysine-rich C-terminal, and no antigenic properties in LysM2 protein. This optimized endolysin revealed impacts against Proteus (sp) by turbidity, and an antibacterial activity against Klebsiella pneumoniae, Salmonella typhimurium, and Proteus vulgaris in agar diffusion assays.
Conclusion: Taken together, our results confirmed that LysM2 is an inhibiting agent for gram-negative bacteria.
Keywords: Antibacterial activity; Bacteriophage SPP1; Endolysin; Siphoviridae.
Copyright© 2022 Avicenna Research Institute.
Conflict of interest statement
Conflict of Interest The authors declare that they have no competing interest.
Figures


References
-
- Scheurwater EM, Pfeffer JM, Clarke AJ. Production and purification of the bacterial autolysin N-acetylmuramoyl-L-alanine amidase B from Pseudomonas aeruginosa. Protein Expr Purif 2007;56(1):128–37. - PubMed
-
- Grishin AV, Karyagina AS, Vasina DV, Vasina IV, Gushchin VA, Lunin VG. Resistance to peptidoglycan-degrading enzymes. Crit Rev Microbiol 2020;46(6):703–26. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
