Developing a conceptual model of symptoms and impacts in progressive fibrosing interstitial lung disease to evaluate patient-reported outcome measures
- PMID: 35509443
- PMCID: PMC9062300
- DOI: 10.1183/23120541.00681-2021
Developing a conceptual model of symptoms and impacts in progressive fibrosing interstitial lung disease to evaluate patient-reported outcome measures
Abstract
Background: An understanding of the experience of patients with progressive fibrosing interstitial lung disease (PF-ILD) is needed to select appropriate patient-reported outcome measures (PROMs) to evaluate treatment effect in clinical trials.
Methods: A systematic literature review was conducted to develop a preliminary conceptual model of the symptoms experienced by patients with PF-ILD and the impacts the disease has on them. An online survey and consensus meetings were then conducted with 12-14 stakeholders (patients, clinicians, regulatory and payer advisors) to refine the conceptual model and critically appraise how key concepts should be measured by PROMs. PROMs assessed included Living with Idiopathic Pulmonary Fibrosis, Living with Pulmonary Fibrosis, the King's Brief Interstitial Lung Disease questionnaire, Cough and Sputum Assessment Questionnaire, Evaluating Respiratory Symptoms, Leicester Cough Questionnaire, Functional Assessment of Chronic Illness Therapy (Dyspnoea/Fatigue) and St George's Respiratory Questionnaire for Idiopathic Pulmonary Fibrosis.
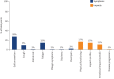
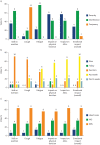
Results: The literature review identified 36 signs/symptoms and 43 impacts directly or indirectly related to pulmonary aspects of PF-ILD. The most relevant symptoms identified by participants included shortness of breath on exertion, fatigue and cough; relevant impacts included effects on physical functioning, activities of daily living and emotional wellbeing. These are presented in a conceptual model. Consensus opinion was that existing PROMs need further modification and validation before use in clinical trials.
Conclusions: The conceptual model improves understanding of the symptoms and impacts that living with PF-ILD has on patients' wellbeing. It can help to inform the choice of PROMs in clinical trials and highlight aspects to assess in the clinical care of patients with PF-ILD.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: M. Wijsenbeek reports receiving support for the present manuscript from Boehringer Ingelheim; grants or contracts received from Hoffmann-La Roche and Boehringer Ingelheim, outside the submitted work; consulting fees received from Roche, Boehringer Ingelheim, Galapagos, Bristol Myers Squibb, Galecto and Respivant, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer Ingelheim, F. Hoffmann-La Roche and Novartis, outside the submitted work; support for attending meetings and/or travel received from Boehringer Ingelheim and F. Hoffmann-La Roche, outside the submitted work; participation on a data safety monitoring or advisory boards for Savara and Galapagos; and is chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, a member of the board of the Netherlands Respiratory Society, a member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and Related Disorders Federation, chair of the educational committee of the European Reference Network for Rare Lung Diseases, and on the advisory board of the Dutch Lung Fibrosis and Sarcoidosis patient associations. Conflict of interest: M. Molina-Molina reports receiving support for the present manuscript from Boehringer Ingelheim; grants received from Roche and Boehringer Ingelheim, outside the submitted work; consulting fees received from Roche, Boehringer Ingelheim, Esteve-Teijin, Pfizer and Chiesi, outside the submitted work. Conflict of interest: O. Chassany reports receiving support for the present manuscript from Boehringer Ingelheim; and consulting fees received from IQVIA, Sanofi, Boiron and ViiV, outside the submitted work. Conflict of interest: J. Fox reports receiving support for the present manuscript from Boehringer Ingelheim. Conflict of interest: L. Galvin reports receiving support for the present manuscript from Boehringer Ingelheim, and is an unpaid director and committee member of the Irish Lung Fibrosis Association, outside the submitted work. Conflict of interest: K. Geissler reports receiving support for the present manuscript from Boehringer Ingelheim. Conflict of interest: K.M. Hammitt reports receiving support for the present manuscript from Boehringer Ingelheim. Novartis paid the Sjogren's Foundation for her to serve on its advisory board although her position does not involve anything pulmonary related. Conflict of interest: M. Kreuter reports receiving support for the present manuscript from Boehringer Ingelheim, and receiving grants or contracts from Boehringer Ingelheim and Roche, outside the submitted work; consulting fees received from Boehringer Ingelheim, Galapagos and Roche, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events received from Boehringer, Galapagos and Roche, outside the submitted work; and participation on data safety monitoring or advisory boards for Boehringer Ingelheim, Galapagos and Roche, outside the submitted work. Conflict of interest: T. Moua reports receiving support for the present manuscript from Boehringer Ingelheim; and grants or contracts received from Boehringer Ingelheim, outside the submitted work. He is an associate editor of this journal. Conflict of interest: E.C. O'Brien reports receiving support for the present manuscript from Boehringer Ingelheim, and consulting fees received from Boehringer Ingelheim outside the submitted work. Conflict of interest: A.F. Slagle reports receiving support for the present manuscript from Boehringer Ingelheim, and receiving consulting fees from Boehringer Ingelheim and IQVIA outside the submitted work. Conflict of interest: A. Krasnow reports receiving support for the present manuscript from Boehringer Ingelheim. The author is a current employee of IQVIA. Conflict of interest: M. Reaney reports receiving support for the present manuscript from Boehringer Ingelheim. The author is a current employee of IQVIA. Conflict of interest: M. Baldwin reports receiving support for the present manuscript from Boehringer Ingelheim. The author is a current employee of Boehringer Ingelheim. Conflict of interest: N. Male reports receiving support for the present manuscript from Boehringer Ingelheim. The author is a current employee of Boehringer Ingelheim. Conflict of interest: K.B. Rohr reports receiving support for the present manuscript from Boehringer Ingelheim. Conflict of interest: J. Swigris reports receiving support for the present manuscript from Boehringer Ingelheim. The author is a current employee of Boehringer Ingelheim. He has received consulting fees from Boehringer Ingelheim, Bristol Myers Squibb, Genentech and Live Fully, outside the submitted work. Conflict of interest: K. Antoniou received support for the present manuscript from Boehringer Ingelheim; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer Ingelheim and Roche, outside the submitted work; support for attending meetings and/or travel received from Boehringer Ingelheim and Roche, outside the submitted work; and is the ERS Assembly 12 Head (unpaid).
Figures