Smurf2 suppresses the metastasis of hepatocellular carcinoma via ubiquitin degradation of Smad2
- PMID: 35509688
- PMCID: PMC8874264
- DOI: 10.1515/med-2022-0437
Smurf2 suppresses the metastasis of hepatocellular carcinoma via ubiquitin degradation of Smad2
Abstract
Purpose: Smurf2, one of C2-WW-HECT domain E3 ubiquitin ligases, is closely related to the development and progression in different cancer types, including hepatocellular carcinoma (HCC). This study aims to illustrate the expression and molecular mechanism of Smurf2 in regulating the progression of HCC.
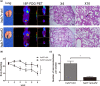
Methods: The expression of Smurf2 in human HCC and adjacent non-tumor liver specimens was detected using tissue microarray studies from 220 HCC patients who underwent curative resection. The relationships of Smurf2 and HCC progression and survival were analyzed using the chi-square test, Kaplan-Meier analysis, and Cox proportional hazards model. For Smurf2 was low expression in HCC cell lines, Smurf2 overexpression cell lines were established. The effect of Smurf2 on cell proliferation and migration was detected by Cell Counting Kit-8 and colony formation assay, and the epithelial-mesenchymal transition (EMT) markers and its transcription factors were tested by immunoblotting. The interaction and ubiquitination of Smad2 by Smurf2 were detected by co-immunoprecipitation and immunoprecipitation assay. Finally, the effect of Smurf2 on HCC was verified using the mouse lung metastasis model.
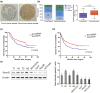
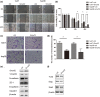
Results: Smurf2 was downregulated in HCC tissues compared to that of corresponding non-tumor liver specimens. The low expression of Smurf2 in HCC was significantly associated with macrovascular or microvascular tumor thrombus and the impairment of overall survival and disease-free survival. In vitro and in vivo analysis showed that Smurf2 overexpression decreased the EMT potential of HCC cells by promoting the ubiquitination of Smad2 via the proteasome-dependent degradation pathway.
Conclusion: The expression of Smurf2 was downregulated in HCC specimens and affected the survival of patients. Smurf2 inhibited the EMT of HCC by enhancing Smad2 ubiquitin-dependent proteasome degradation.
Keywords: Smad2; Smurf2; hepatocellular carcinoma; metastasis; ubiquitination.
© 2022 Dongqiang Song et al., published by De Gruyter.
Conflict of interest statement
Conflict of interest: The author reports no conflicts of interest in this work.
Figures


References
-
- Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinicians. 2021;71:209–49. - PubMed
- Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinicians. 2021;71:209–49. - PubMed
-
- Sutter SA, Slinker A, Balumuka DD, Mitchell KB. Surgical management of breast cancer in Africa: a continent-wide review of intervention practices, barriers to care, and adjuvant therapy. J Glob Oncol. 2017;3:162–8. - PMC - PubMed
- Sutter SA, Slinker A, Balumuka DD, Mitchell KB. Surgical management of breast cancer in Africa: a continent-wide review of intervention practices, barriers to care, and adjuvant therapy. J Glob Oncol. 2017;3:162–8. - PMC - PubMed
-
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncology: Off J Eur Soc Med Oncol. 2019;30:871–3. - PubMed
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncology: Off J Eur Soc Med Oncol. 2019;30:871–3. - PubMed
-
- Kumari N, Jaynes PW, Saei A, Iyengar PV, Richard JLC, Eichhorn PJA. The roles of ubiquitin modifying enzymes in neoplastic disease. Biochimica et Biophysica Acta Rev cancer. 2017;1868:456–83. - PubMed
- Kumari N, Jaynes PW, Saei A, Iyengar PV, Richard JLC, Eichhorn PJA. The roles of ubiquitin modifying enzymes in neoplastic disease. Biochimica et Biophysica Acta Rev cancer. 2017;1868:456–83. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
