Seahorse Protein Hydrolysate Ameliorates Proinflammatory Mediators and Cartilage Degradation on Posttraumatic Osteoarthritis with an Obesity Rat Model
- PMID: 35509713
- PMCID: PMC9060998
- DOI: 10.1155/2022/4117520
Seahorse Protein Hydrolysate Ameliorates Proinflammatory Mediators and Cartilage Degradation on Posttraumatic Osteoarthritis with an Obesity Rat Model
Abstract
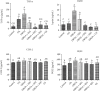
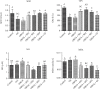
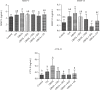
Osteoarthritis (OA) is one of the age-related diseases and is highly present on the knees. Obesity and mechanical injuries as a risk factor of OA are attributed to cartilage disintegration, joint loading, and inflammation. This study is aimed at investigating the effects of seahorse protein hydrolysate (SH) on posttraumatic osteoarthritis in an obesity rat. The OA model was developed by anterior cruciate ligament transection with medial meniscectomy in a high-fat diet- (HFD-) induced obesity rat model. The male Sprague-Dawley rats were fed a HFD for 6 weeks before OA surgery. The OA rats were treated with oral gavage by 4, 8, or 20 mg/kg of body weight of SH for 6 weeks of treatment. The expressions of plasma proinflammatory factors, C-telopeptide of type II collagen, and matrix metalloproteinase- (MMP-) 3 and MMP-13 were reduced by SH treatment. Plasma superoxide dismutase and glutathione peroxidase activities were enhanced by SH. SH also relieved the pain of the knee joint and swelling as well as decreased proteoglycan loss in the knee articular cartilage caused by osteoarthritis. Based on these results, SH suppressed proinflammatory factors and attenuated cartilage degradation and pain in the OA model. Therefore, seahorse protein hydrolysate might be a potential opportunity for improving the development of osteoarthritis.
Copyright © 2022 Sabri Sudirman et al.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures

References
-
- Prieto-Alhambra D., Judge A., Javaid M. K., Cooper C., Diez-Perez A., Arden N. K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Annals of the Rheumatic Diseases . 2014;73(9):1659–1664. doi: 10.1136/annrheumdis-2013-203355. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

