In vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications
- PMID: 35510408
- PMCID: PMC9545022
- DOI: 10.1111/jce.15522
In vivo porcine characterization of atrial lesion safety and efficacy utilizing a circular pulsed-field ablation catheter including assessment of collateral damage to adjacent tissue in supratherapeutic ablation applications
Abstract
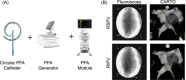
Introduction: Pulsed-field ablation (PFA), an ablative method that causes cell death by irreversible electroporation, has potential safety advantages over radiofrequency ablation and cryoablation. Pulmonary vein (PV) isolation was performed in a porcine model to characterize safety and performance of a novel, fully-integrated biphasic PFA system comprising a multi-channel generator, variable loop circular catheter, and integrated PFA mapping software module.
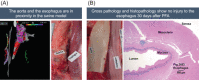
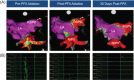
Methods: Eight healthy porcine subjects were included. To evaluate safety, multiple ablations were performed, including sites not generally targeted for therapeutic ablation, such as the right inferior PV lumen, right superior PV ostium, and adjacent to the esophagus and phrenic nerve. To evaluate the efficacy, animals were recovered, followed for 30(±3) days, then re-mapped. Gross pathological and histopathological examinations assessed procedural injuries, chronic thrombosis, tissue ablation, penetration depth, healing, and inflammatory response.
Results: All eight animals survived follow-up. PV narrowing was not observed acutely nor at follow-up, even when ablation was performed deep to the PV ostium. No injury was seen grossly or histologically in adjacent structures. All PVs were durably isolated, confirmed by bidirectional block at re-map procedure. Histological examination showed complete, transmural necrosis around the circumference of the ablated section of right PVs.
Conclusion: This preclinical evaluation of a fully-integrated PFA system demonstrated effective and durable ablation of cardiac tissue and PV isolation without collateral damage to adjacent structures, even when ablation was performed in more extreme settings than those used therapeutically. Histological staining confirmed complete transmural cell necrosis around the circumference of the PV ostium at 30 days.
Keywords: catheter ablation; irreversible electroporation; preclinical model; pulmonary vein isolation; pulsed-field ablation.
© 2022 The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Figures



Comment in
-
Pulsed-field ablation: What are the unknowns and when will they cease to concern us?J Cardiovasc Electrophysiol. 2022 Jul;33(7):1489-1493. doi: 10.1111/jce.15521. Epub 2022 May 25. J Cardiovasc Electrophysiol. 2022. PMID: 35510406 No abstract available.
References
-
- Muthalaly RG, John RM, Schaeffer B, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:854‐860. - PubMed
-
- Apte NM, Shrestha A, Dendi R. Correction to: techniques to avoid complications of atrial fibrillation ablation. Curr Treat Options Cardiovasc Med. 2020;22:37.
-
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223‐231. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

