A multi-hit hypothesis for an APOE4-dependent pathophysiological state
- PMID: 35510513
- PMCID: PMC9796338
- DOI: 10.1111/ejn.15685
A multi-hit hypothesis for an APOE4-dependent pathophysiological state
Abstract
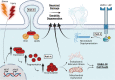
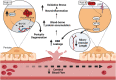
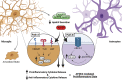
The APOE gene encoding the Apolipoprotein E protein is the single most significant genetic risk factor for late-onset Alzheimer's disease. The APOE4 genotype confers a significantly increased risk relative to the other two common genotypes APOE3 and APOE2. Intriguingly, APOE4 has been associated with neuropathological and cognitive deficits in the absence of Alzheimer's disease-related amyloid or tau pathology. Here, we review the extensive literature surrounding the impact of APOE genotype on central nervous system dysfunction, focussing on preclinical model systems and comparison of APOE3 and APOE4, given the low global prevalence of APOE2. A multi-hit hypothesis is proposed to explain how APOE4 shifts cerebral physiology towards pathophysiology through interconnected hits. These hits include the following: neurodegeneration, neurovascular dysfunction, neuroinflammation, oxidative stress, endosomal trafficking impairments, lipid and cellular metabolism disruption, impaired calcium homeostasis and altered transcriptional regulation. The hits, individually and in combination, leave the APOE4 brain in a vulnerable state where further cumulative insults will exacerbate degeneration and lead to cognitive deficits in the absence of Alzheimer's disease pathology and also a state in which such pathology may more easily take hold. We conclude that current evidence supports an APOE4 multi-hit hypothesis, which contributes to an APOE4 pathophysiological state. We highlight key areas where further study is required to elucidate the complex interplay between these individual mechanisms and downstream consequences, helping to frame the current landscape of existing APOE-centric literature.
Keywords: APOE; ageing; multihit hypothesis; pathophysiology; review.
© 2022 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





References
-
- Andrews‐Zwilling, Y. , Bien‐Ly, N. , Xu, Q. , Li, G. , Bernardo, A. , Yoon, S. Y. , Zwilling, D. , Yan, T. X. , Chen, L. , & Huang, Y. (2010). Apolipoprotein E4 causes age‐ and Tau‐dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. Journal of Neuroscience, 30(41), 13707–13717. 10.1523/JNEUROSCI.4040-10.2010 - DOI - PMC - PubMed
-
- Area‐Gomez, E. , Larrea, D. , Pera, M. , Agrawal, R. R. , Guilfoyle, D. N. , Pirhaji, L. , Shannon, K. , Arain, H. A. , Ashok, A. , Chen, Q. , Dillman, A. A. , Figueroa, H. Y. , Cookson, M. R. , Gross, S. S. , Fraenkel, E. , Duff, K. E. , & Nuriel, T. (2020). APOE4 is associated with differential regional vulnerability to bioenergetic deficits in aged APOE mice. Scientific Reports, 10(1), 1–20. 10.1038/s41598-020-61142-8 - DOI - PMC - PubMed
-
- Arnold, M. , Nho, K. , Kueider‐Paisley, A. , Massaro, T. , Huynh, K. , Brauner, B. , MahmoudianDehkordi, S. , Louie, G. , Moseley, M. A. , Thompson, J. W. , John‐Williams, L. S. , Tenenbaum, J. D. , Blach, C. , Chang, R. , Brinton, R. D. , Baillie, R. , Han, X. , Trojanowski, J. Q. , Shaw, L. M. , … Kastenmüller, G. (2020). Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome. Nature Communications, 11(1), 1148. https://www.nature.com/articles/s41467-020-14959-w, 10.1038/s41467-020-14959-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

