Curcumol enhances cisplatin sensitivity of gastric cancer: involvement of microRNA-7 and the nuclear factor-kappa B/snail family transcriptional repressor 1 axis
- PMID: 35510522
- PMCID: PMC9275945
- DOI: 10.1080/21655979.2022.2070975
Curcumol enhances cisplatin sensitivity of gastric cancer: involvement of microRNA-7 and the nuclear factor-kappa B/snail family transcriptional repressor 1 axis
Abstract
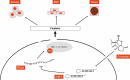
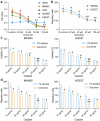
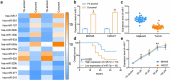
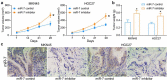
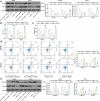
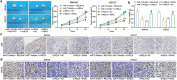
Cisplatin is a primary chemotherapeutic drug for gastric cancer (GC) patients, but the drug resistance remains the leading cause of treatment failure and high mortality. Curcumol is a bioactive sesquiterpenoid that has reportedly been linked to cisplatin sensitivity in GC. This study focuses on the exact functions of curcumol in the cisplatin sensitivity of GC cells and the molecules of action. The curcumol treatment reduced the viability and migration and enhanced cisplatin sensitivity of GC cells in a dose-dependent manner. Microarray analysis suggested that microRNA-7 (miR-7) was the most upregulated miRNA in GC cells after curcumol treatment. The Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis showed that the curcumol-affected genes, including the target genes of miR-7, were enriched in the nuclear factor-kappa B (NF-κB) pathway, whose activity was suppressed after curcumol treatment. miR-7 was found to target and suppress RELA proto-oncogene (RELA, also known as p65), a NF-κB subunit. Downregulation of miR-7 blocked the sensitizing effects of curcumol on cells to cisplatin and led to increased expression of NF-κB p65 and snail family transcriptional repressor 1 (SNAIL). Further downregulation of RELA enhanced, whereas upregulation of SNAIL suppressed the sensitivity again. In summary, this study suggests that curcumol sensitizes GC cells to cisplatin via miR-7 and the suppression of the NF-κB/SNAIL axis. The findings may offer new thoughts that curcumol in combination with cisplatin might be a useful strategy for GC management.
Keywords: Curcumol; NF-κB/SNAIL; RELA; cisplatin; gastric cancer; miR-7.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
