Challenges in the use of sortase and other peptide ligases for site-specific protein modification
- PMID: 35510539
- PMCID: PMC9126251
- DOI: 10.1039/d0cs01148g
Challenges in the use of sortase and other peptide ligases for site-specific protein modification
Abstract
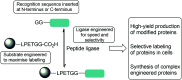
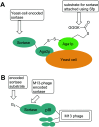
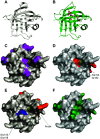
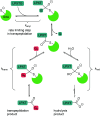
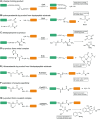
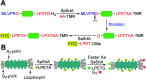
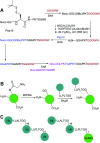
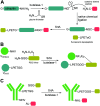
Site-specific protein modification is a widely-used biochemical tool. However, there are many challenges associated with the development of protein modification techniques, in particular, achieving site-specificity, reaction efficiency and versatility. The engineering of peptide ligases and their substrates has been used to address these challenges. This review will focus on sortase, peptidyl asparaginyl ligases (PALs) and variants of subtilisin; detailing how their inherent specificity has been utilised for site-specific protein modification. The review will explore how the engineering of these enzymes and substrates has led to increased reaction efficiency mainly due to enhanced catalytic activity and reduction of reversibility. It will also describe how engineering peptide ligases to broaden their substrate scope is opening up new opportunities to expand the biochemical toolkit, particularly through the development of techniques to conjugate multiple substrates site-specifically onto a protein using orthogonal peptide ligases.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

