Midgestation Leptin Infusion Induces Characteristics of Clinical Preeclampsia in Mice, Which Is Ablated by Endothelial Mineralocorticoid Receptor Deletion
- PMID: 35510543
- PMCID: PMC9177735
- DOI: 10.1161/HYPERTENSIONAHA.121.18832
Midgestation Leptin Infusion Induces Characteristics of Clinical Preeclampsia in Mice, Which Is Ablated by Endothelial Mineralocorticoid Receptor Deletion
Abstract
Background: Patients with preeclampsia demonstrate increases in placental leptin production in midgestation, and an associated increase in late gestation plasma leptin levels. The consequences of mid-late gestation increases in leptin production in pregnancy is unknown. Our previous work indicates that leptin infusion induces endothelial dysfunction in nonpregnant female mice via leptin-mediated aldosterone production and endothelial mineralocorticoid receptor (ECMR) activation, which is ablated by ECMR deletion. Therefore, we hypothesized that leptin infusion in mid-gestation of pregnancy induces endothelial dysfunction and hypertension, hallmarks of clinical preeclampsia, which are prevented by ECMR deletion.
Methods: Leptin was infused via miniosmotic pump (0.9 mg/kg per day) into timed-pregnant ECMR-intact (WT) and littermate-mice with ECMR deletion (KO) on gestation day (GD)11-18.
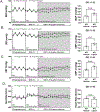
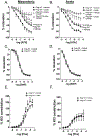
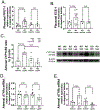
Results: Leptin infusion decreased fetal weight and placental efficiency in WT mice compared with WT+vehicle. Radiotelemetry recording demonstrated that blood pressure increased in leptin-infused WT mice during infusion. Leptin infusion reduced endothelial-dependent relaxation responses to acetylcholine (ACh) in both resistance (second-order mesenteric) and conduit (aorta) vessels in WT pregnant mice. Leptin infusion increased placental ET-1 (endothelin-1) production evidenced by increased PPET-1 (preproendothelin-1) and ECE-1 (endothelin-converting enzyme-1) expressions in WT mice. Adrenal aldosterone synthase (CYP11B2) and angiotensin II type 1 receptor b (AT1Rb) expression increased with leptin infusion in pregnant WT mice. KO pregnant mice demonstrated protection from leptin-induced reductions in pup weight, placental efficiency, increased BP, and endothelial dysfunction.
Conclusions: Collectively, these data indicate that leptin infusion in midgestation induces endothelial dysfunction, hypertension, and fetal growth restriction in pregnant mice, which is ablated by ECMR deletion.
Keywords: endothelial function; hypertension; leptin; mice; preeclampsia.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest
Figures
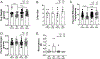


Similar articles
-
Both endothelial mineralocorticoid receptor expression and hyperleptinemia are required for clinical characteristics of placental ischemia in mice.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H118-H130. doi: 10.1152/ajpheart.00188.2024. Epub 2024 May 17. Am J Physiol Heart Circ Physiol. 2024. PMID: 38758130 Free PMC article.
-
Selective deletion of endothelial mineralocorticoid receptor protects from vascular dysfunction in sodium-restricted female mice.Biol Sex Differ. 2020 Nov 23;11(1):64. doi: 10.1186/s13293-020-00340-5. Biol Sex Differ. 2020. PMID: 33228767 Free PMC article.
-
Mid-late gestation leptin infusion induces placental mitochondrial and endoplasmic reticulum unfolded protein responses in a mouse model of preeclampsia.Placenta. 2024 Dec;158:253-262. doi: 10.1016/j.placenta.2024.11.001. Epub 2024 Nov 2. Placenta. 2024. PMID: 39522465
-
Mechanisms of leptin-induced endothelial dysfunction.Curr Opin Nephrol Hypertens. 2023 Mar 1;32(2):118-123. doi: 10.1097/MNH.0000000000000867. Epub 2022 Dec 29. Curr Opin Nephrol Hypertens. 2023. PMID: 36598435 Free PMC article. Review.
-
Emerging Role of Leptin in Vascular and Placental Dysfunction in Preeclampsia.Arterioscler Thromb Vasc Biol. 2025 May;45(5):585-599. doi: 10.1161/ATVBAHA.124.321676. Epub 2025 Apr 3. Arterioscler Thromb Vasc Biol. 2025. PMID: 40177777 Review.
Cited by
-
Insulin Elevates ID2 Expression in Trophoblasts and Aggravates Preeclampsia in Obese ASB4-Null Mice.Int J Mol Sci. 2023 Jan 21;24(3):2149. doi: 10.3390/ijms24032149. Int J Mol Sci. 2023. PMID: 36768469 Free PMC article.
-
CD4+ T Cells Expressing Viral Proteins Induce HIV-Associated Endothelial Dysfunction and Hypertension Through Interleukin 1α-Mediated Increases in Endothelial NADPH Oxidase 1.Circulation. 2025 Apr 22;151(16):1187-1203. doi: 10.1161/CIRCULATIONAHA.124.070538. Epub 2025 Feb 5. Circulation. 2025. PMID: 39907014
-
Both endothelial mineralocorticoid receptor expression and hyperleptinemia are required for clinical characteristics of placental ischemia in mice.Am J Physiol Heart Circ Physiol. 2024 Jul 1;327(1):H118-H130. doi: 10.1152/ajpheart.00188.2024. Epub 2024 May 17. Am J Physiol Heart Circ Physiol. 2024. PMID: 38758130 Free PMC article.
-
Excessive Pregestational Weight and Maternal Obstetric Complications: The Role of Adipokines.Int J Mol Sci. 2023 Sep 28;24(19):14678. doi: 10.3390/ijms241914678. Int J Mol Sci. 2023. PMID: 37834125 Free PMC article. Review.
-
12-week Dolutegravir treatment marginally reduces energy expenditure but does not increase body weight or alter vascular function in a murine model of Human Immunodeficiency Virus infection.Vascul Pharmacol. 2024 Jun;155:107288. doi: 10.1016/j.vph.2024.107288. Epub 2024 Feb 28. Vascul Pharmacol. 2024. PMID: 38428626 Free PMC article.
References
-
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, Wolff T, Steiner CA, Elixhauser A. Delivery hospitalizations involving preeclampsia and eclampsia, 2005-2014: Statistical brief #222. Healthcare cost and utilization project (hcup) statistical briefs. Rockville (MD); 2006. - PubMed
-
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

