Electrochemistry and Reactivity of Chelation-stabilized Hypervalent Bromine(III) Compounds
- PMID: 35510557
- PMCID: PMC9401590
- DOI: 10.1002/chem.202200974
Electrochemistry and Reactivity of Chelation-stabilized Hypervalent Bromine(III) Compounds
Abstract
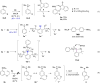
Hypervalent bromine(III) reagents possess a higher electrophilicity and a stronger oxidizing power compared to their iodine(III) counterparts. Despite the superior reactivity, bromine(III) reagents have a reputation of hard-to-control and difficult-to-synthesize compounds. This is partly due to their low stability, and partly because their synthesis typically relies on the use of the toxic and highly reactive BrF3 as a precursor. Recently, we proposed chelation-stabilized hypervalent bromine(III) compounds as a possible solution to both problems. First, they can be conveniently prepared by electro-oxidation of the corresponding bromoarenes. Second, the chelation endows bromine(III) species with increased stability while retaining sufficient reactivity, comparable to that of iodine(III) counterparts. Finally, their intrinsic reactivity can be unlocked in the presence of acids. Herein, an in-depth mechanistic study of both the electrochemical generation and the reactivity of the bromine(III) compounds is disclosed, with implications for known applications and future developments in the field.
Keywords: bromane; cyclic voltammetry; hypervalent halogen; oxidative coupling; unified pH.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



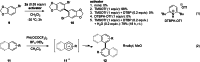

Similar articles
-
Electrochemical Generation of Hypervalent Bromine(III) Compounds.Angew Chem Int Ed Engl. 2021 Jul 12;60(29):15832-15837. doi: 10.1002/anie.202104677. Epub 2021 Jun 9. Angew Chem Int Ed Engl. 2021. PMID: 33894098 Free PMC article.
-
Predicting bond dissociation energies of cyclic hypervalent halogen reagents using DFT calculations and graph attention network model.Beilstein J Org Chem. 2024 Jun 28;20:1444-1452. doi: 10.3762/bjoc.20.127. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38952960 Free PMC article.
-
Cyclic Hypervalent Iodine Reagents: Enabling Tools for Bond Disconnection via Reactivity Umpolung.Acc Chem Res. 2018 Dec 18;51(12):3212-3225. doi: 10.1021/acs.accounts.8b00468. Epub 2018 Nov 28. Acc Chem Res. 2018. PMID: 30485071
-
[Development of Hypervalent Iodine Reagents Utilizing Functional Group Properties].Yakugaku Zasshi. 2025;145(5):387-393. doi: 10.1248/yakushi.25-00005. Yakugaku Zasshi. 2025. PMID: 40307022 Review. Japanese.
-
Non-Palladium-Catalyzed Oxidative Coupling Reactions Using Hypervalent Iodine Reagents.Front Chem. 2022 Jul 1;10:909250. doi: 10.3389/fchem.2022.909250. eCollection 2022. Front Chem. 2022. PMID: 35844643 Free PMC article. Review.
Cited by
-
Electrochemical Synthesis of Dimeric λ3-Bromane: Platform for Hypervalent Bromine(III) Compounds.Org Lett. 2023 Mar 31;25(12):2047-2052. doi: 10.1021/acs.orglett.3c00405. Epub 2023 Mar 21. Org Lett. 2023. PMID: 36944352 Free PMC article.
-
Diaryl hypervalent bromines and chlorines: synthesis, structures and reactivities.Chem Sci. 2024 Jan 3;15(5):1557-1569. doi: 10.1039/d3sc05382b. eCollection 2024 Jan 31. Chem Sci. 2024. PMID: 38303936 Free PMC article. Review.
-
Iodine-Iodine Cooperation Enables Metal-Free C-N Bond-Forming Electrocatalysis via Isolable Iodanyl Radicals.J Am Chem Soc. 2022 Aug 3;144(30):13913-13919. doi: 10.1021/jacs.2c05562. Epub 2022 Jul 20. J Am Chem Soc. 2022. PMID: 35856717 Free PMC article.
-
Highly Acidic Electron-Rich Brønsted Acids Accelerate Asymmetric Pictet-Spengler Reactions by Virtue of Stabilizing Cation-π Interactions.J Am Chem Soc. 2024 Oct 3;146(41):28339-49. doi: 10.1021/jacs.4c09421. Online ahead of print. J Am Chem Soc. 2024. PMID: 39361889 Free PMC article.
-
Multifunctional Organocatalysts - Singly-Linked and Macrocyclic Bisphosphoric Acids for Asymmetric Phase-Transfer and Brønsted-Acid Catalysis.Chemistry. 2023 Jan 9;29(2):e202202953. doi: 10.1002/chem.202202953. Epub 2022 Nov 7. Chemistry. 2023. PMID: 36161384 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

