Refractory immune TTP following Pfizer-BioNTech COVID-19 vaccine successfully salvaged with caplacizumab
- PMID: 35510743
- PMCID: PMC9347500
- DOI: 10.1111/jth.15751
Refractory immune TTP following Pfizer-BioNTech COVID-19 vaccine successfully salvaged with caplacizumab
Abstract
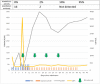
Immune thrombotic thrombocytopenic purpura (iTTP) is a life-threatening thrombotic microangiopathy caused by antibodies against ADAMTS13. We report a young, healthy female who developed hematuria, vomiting, and hematemesis 3 weeks after her first dose of Pfizer Bio-NTech COVID-19 vaccine. Investigations confirmed iTTP with undetectable ADAMTS13 activity and a positive antibody assay. Despite initial response to standard treatment with plasma exchange and corticosteroids, she had an acute deterioration of her TTP with neurological and cardiac involvement. Fortunately, she then had prompt response to rituximab and emergently obtained caplacizumab and is now in remission. Although most cases of iTTP are of unknown etiology, we cannot exclude that her almost fatal iTTP episode was triggered by the Pfizer-BioNTech COVID-19 vaccine. This case also highlights the ability of caplacizumab to quickly halt disseminated thrombus formation in refractory TTP.
Keywords: ADAMTS13 protein; COVID 19 vaccines; caplacizumab; plasma exchange; thrombotic thrombocytopenic purpura.
© 2022 International Society on Thrombosis and Haemostasis.
Figures
References
-
- Scully M., Hunt B.J., Benjamin S., et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(3):323–335. - PubMed
-
- Scully M., Cataland S.R., Peyvandi F., et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical