Plant growth-promoting rhizobacterium Pseudomonas sp. CM11 specifically induces lateral roots
- PMID: 35510807
- PMCID: PMC9546010
- DOI: 10.1111/nph.18199
Plant growth-promoting rhizobacterium Pseudomonas sp. CM11 specifically induces lateral roots
Abstract
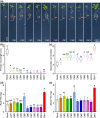
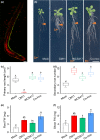
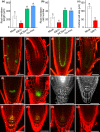
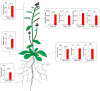
Plant growth-promoting rhizobacteria are involved in altering secondary root (SR) formation, but hitherto there has been no distinction between the different types of SRs upon induction of soil biota, and the genetic pathways involved. By using plate and soil systems, we studied the effects of the Pseudomonas strains CM11 and WCS417 on plant performance with a focus on root development. Through a combination of cellular, molecular and genetic analyses, we investigated the type of SRs induced upon CM11 and WCS417 root inoculation using genetic pathways associated with specific SR types. CM11 was shown to affect the root architecture differently from WCS417. CM11 inoculation leads to primary root arrest, whereas WCS417 reveals a longer primary root. Both CM11 and WCS417 activate the PLETHORA 3,5,7-controlled lateral root pathway, rather than the WUSCHEL-RELATED HOMEOBOX 11,12-controlled adventitious (lateral) root pathway. In addition, CM11 promotes plant growth in model and various crop species. It improves plant fitness traits, such as bigger shoots, faster bolting and higher yield in terms of seeds. Our results indicate that the root system architecture can be promoted by activation of PLETHORA 3,5,7 dependent primed lateral pre-branch sites upon inoculation with CM11, which creates great potential to gain a better understanding of root plasticity.
Keywords: Pseudomonas; PLETHORA; Rhizobacteria; lateral roots; plant growth-promoting; root architecture; secondary roots.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Figures

Similar articles
-
Cell-type-specific transcriptomics reveals that root hairs and endodermal barriers play important roles in beneficial plant-rhizobacterium interactions.Mol Plant. 2023 Jul 3;16(7):1160-1177. doi: 10.1016/j.molp.2023.06.001. Epub 2023 Jun 5. Mol Plant. 2023. PMID: 37282370 Free PMC article.
-
Rhizobacteria-Mediated Activation of the Fe Deficiency Response in Arabidopsis Roots: Impact on Fe Status and Signaling.Front Plant Sci. 2019 Jul 12;10:909. doi: 10.3389/fpls.2019.00909. eCollection 2019. Front Plant Sci. 2019. PMID: 31354776 Free PMC article.
-
Arabidopsis thaliana Early Foliar Proteome Response to Root Exposure to the Rhizobacterium Pseudomonas simiae WCS417.Mol Plant Microbe Interact. 2023 Nov;36(11):737-748. doi: 10.1094/MPMI-05-23-0071-R. Epub 2023 Nov 27. Mol Plant Microbe Interact. 2023. PMID: 37470457
-
Mechanism of plant growth promotion by rhizobacteria.Indian J Exp Biol. 2000 Sep;38(9):856-62. Indian J Exp Biol. 2000. PMID: 12561941 Review.
-
Root penetration ability and plant growth in agroecosystems.Plant Physiol Biochem. 2022 Jul 15;183:160-168. doi: 10.1016/j.plaphy.2022.04.024. Epub 2022 May 7. Plant Physiol Biochem. 2022. PMID: 35605464 Review.
Cited by
-
Unveiling the multifaceted potential of Pseudomonas khavaziana strain SR9: a promising biocontrol agent for wheat crown rot.Microbiol Spectr. 2024 Oct 3;12(10):e0071224. doi: 10.1128/spectrum.00712-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162535 Free PMC article.
-
Advances in understanding cold tolerance in grapevine.Plant Physiol. 2023 Jul 3;192(3):1733-1746. doi: 10.1093/plphys/kiad092. Plant Physiol. 2023. PMID: 36789447 Free PMC article. Review.
-
Mechanism on the promotion of host growth and enhancement of salt tolerance by Bacillaceae isolated from the rhizosphere of Reaumuria soongorica.Front Microbiol. 2024 May 31;15:1408622. doi: 10.3389/fmicb.2024.1408622. eCollection 2024. Front Microbiol. 2024. PMID: 38881656 Free PMC article.
-
4-methylumbelliferone (4-MU) enhances drought tolerance of apple by regulating rhizosphere microbial diversity and root architecture.Hortic Res. 2023 May 19;10(6):uhad099. doi: 10.1093/hr/uhad099. eCollection 2023 Jun. Hortic Res. 2023. PMID: 37427035 Free PMC article.
-
Enterobacter-inoculation altered the C, N contents and regulated biomass allocation in Reaumuria soongorica to promote plant growth and improve salt stress tolerance.Front Plant Sci. 2025 Jan 3;15:1502659. doi: 10.3389/fpls.2024.1502659. eCollection 2024. Front Plant Sci. 2025. PMID: 39830945 Free PMC article.
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R et al. 2003. Genome‐wide insertional mutagenesis of Arabidopsis thaliana . Science 301: 653–657. - PubMed
-
- Beeckman T, Burssens S, Inzé D. 2001. The peri‐cell‐cycle in Arabidopsis. Journal of Experimental Botany 52: 403–411. - PubMed
-
- Beeckman T, De Smet I. 2014. Pericycle. Current Biology 24: R378–R379. - PubMed
-
- Bellini C, Pacurar DI, Perrone I. 2014. Adventitious roots and lateral roots: similarities and differences. Annual Review of Plant Biology 65: 639–666. - PubMed
-
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. 2003. Local, efflux‐dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

