A Computational Framework for Atrioventricular Valve Modeling Using Open-Source Software
- PMID: 35510823
- PMCID: PMC9254695
- DOI: 10.1115/1.4054485
A Computational Framework for Atrioventricular Valve Modeling Using Open-Source Software
Abstract
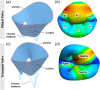
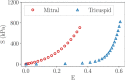
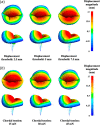
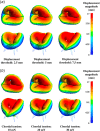
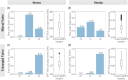
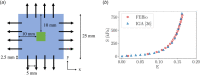
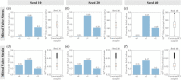
Atrioventricular valve regurgitation is a significant cause of morbidity and mortality in patients with acquired and congenital cardiac valve disease. Image-derived computational modeling of atrioventricular valves has advanced substantially over the last decade and holds particular promise to inform valve repair in small and heterogeneous populations, which are less likely to be optimized through empiric clinical application. While an abundance of computational biomechanics studies has investigated mitral and tricuspid valve disease in adults, few studies have investigated its application to vulnerable pediatric and congenital heart populations. Further, to date, investigators have primarily relied upon a series of commercial applications that are neither designed for image-derived modeling of cardiac valves nor freely available to facilitate transparent and reproducible valve science. To address this deficiency, we aimed to build an open-source computational framework for the image-derived biomechanical analysis of atrioventricular valves. In the present work, we integrated an open-source valve modeling platform, SlicerHeart, and an open-source biomechanics finite element modeling software, FEBio, to facilitate image-derived atrioventricular valve model creation and finite element analysis. We present a detailed verification and sensitivity analysis to demonstrate the fidelity of this modeling in application to three-dimensional echocardiography-derived pediatric mitral and tricuspid valve models. Our analyses achieved an excellent agreement with those reported in the literature. As such, this evolving computational framework offers a promising initial foundation for future development and investigation of valve mechanics, in particular collaborative efforts targeting the development of improved repairs for children with congenital heart disease.
Keywords: atrioventricular valves; contact potential; finite element modeling; open-source; uncertainty analysis; valve mechanics.
Copyright © 2022 by ASME.
Figures










Similar articles
-
Visualization and Quantification of the Unrepaired Complete Atrioventricular Canal Valve Using Open-Source Software.J Am Soc Echocardiogr. 2022 Sep;35(9):985-996.e11. doi: 10.1016/j.echo.2022.04.015. Epub 2022 May 7. J Am Soc Echocardiogr. 2022. PMID: 35537615 Free PMC article.
-
FEBio FINESSE: An Open-Source Finite Element Simulation Approach to Estimate In Vivo Heart Valve Strains Using Shape Enforcement.Ann Biomed Eng. 2025 Jan;53(1):241-259. doi: 10.1007/s10439-024-03637-3. Epub 2024 Nov 5. Ann Biomed Eng. 2025. PMID: 39499365
-
Towards patient-specific modeling of mitral valve repair: 3D transesophageal echocardiography-derived parameter estimation.Med Image Anal. 2017 Jan;35:599-609. doi: 10.1016/j.media.2016.09.006. Epub 2016 Sep 27. Med Image Anal. 2017. PMID: 27718462
-
SlicerHeart: An open-source computing platform for cardiac image analysis and modeling.Front Cardiovasc Med. 2022 Sep 6;9:886549. doi: 10.3389/fcvm.2022.886549. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36148054 Free PMC article. Review.
-
Surgical considerations for atrial functional regurgitation of the mitral and tricuspid valves based on the etiological mechanism.Gen Thorac Cardiovasc Surg. 2021 Jul;69(7):1041-1049. doi: 10.1007/s11748-021-01629-x. Epub 2021 May 10. Gen Thorac Cardiovasc Surg. 2021. PMID: 33970433 Free PMC article. Review.
Cited by
-
Tricuspid Valve Regurgitation in Hypoplastic Left Heart Syndrome: Current Insights and Future Perspectives.J Cardiovasc Dev Dis. 2023 Mar 7;10(3):111. doi: 10.3390/jcdd10030111. J Cardiovasc Dev Dis. 2023. PMID: 36975875 Free PMC article. Review.
-
A noninvasive method for determining elastic parameters of valve tissue using physics-informed neural networks.Acta Biomater. 2025 Jun 15;200:283-298. doi: 10.1016/j.actbio.2025.05.021. Epub 2025 May 26. Acta Biomater. 2025. PMID: 40436231
-
Experimental Assessment of Traction Force and Associated Fetal Brain Deformation in Vacuum-Assisted Delivery.Ann Biomed Eng. 2025 Apr;53(4):825-844. doi: 10.1007/s10439-024-03665-z. Epub 2024 Dec 22. Ann Biomed Eng. 2025. PMID: 39710825 Free PMC article.
-
Bayesian Optimization-Based Inverse Finite Element Analysis for Atrioventricular Heart Valves.Ann Biomed Eng. 2024 Mar;52(3):611-626. doi: 10.1007/s10439-023-03408-6. Epub 2023 Nov 21. Ann Biomed Eng. 2024. PMID: 37989903 Free PMC article.
-
Identifying Heterogeneous Micromechanical Properties of Biological Tissues via Physics-Informed Neural Networks.Small Methods. 2025 Jan;9(1):e2400620. doi: 10.1002/smtd.202400620. Epub 2024 Aug 1. Small Methods. 2025. PMID: 39091065
References
-
- Salgo, I. S. , Gorman, J. H. , Gorman, R. C. , Jackson, B. M. , Bowen, F. W. , Plappert, T. , St John Sutton, M. G. , and Edmunds, L. H. , 2002, “ Effect of Annular Shape on Leaflet Curvature in Reducing Mitral Leaflet Stress,” Circulation, 106(6), pp. 711–717.10.1161/01.CIR.0000025426.39426.83 - DOI - PubMed
-
- Levack, M. M. , Jassar, A. S. , Shang, E. K. , Vergnat, M. , Woo, Y. J. , Acker, M. A. , Jackson, B. M. , Gorman, J. H. , and Gorman, R. C. , 2012, “ Three-Dimensional Echocardiographic Analysis of Mitral Annular Dynamics,” Circulation, 126(11_suppl_1), pp. S183–S188.10.1161/CIRCULATIONAHA.111.084483 - DOI - PMC - PubMed
-
- Lee, C. H. , Oomen, P. J. , Rabbah, J. P. , Yoganathan, A. , Gorman, R. C. , Gorman, J. H. , Amini, R. , and Sacks, M. S. , 2013, “ A High-Fidelity and Micro-Anatomically Accurate 3D Finite Element Model for Simulations of Functional Mitral Valve,” LNCS, 7945, pp. 416–424.10.1007/978-3-642-38899-6_49 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

