Using positional information to provide context for biological image analysis with MorphoGraphX 2.0
- PMID: 35510843
- PMCID: PMC9159754
- DOI: 10.7554/eLife.72601
Using positional information to provide context for biological image analysis with MorphoGraphX 2.0
Abstract
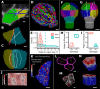
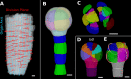
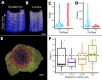
Positional information is a central concept in developmental biology. In developing organs, positional information can be idealized as a local coordinate system that arises from morphogen gradients controlled by organizers at key locations. This offers a plausible mechanism for the integration of the molecular networks operating in individual cells into the spatially coordinated multicellular responses necessary for the organization of emergent forms. Understanding how positional cues guide morphogenesis requires the quantification of gene expression and growth dynamics in the context of their underlying coordinate systems. Here, we present recent advances in the MorphoGraphX software (Barbier de Reuille et al., 2015) that implement a generalized framework to annotate developing organs with local coordinate systems. These coordinate systems introduce an organ-centric spatial context to microscopy data, allowing gene expression and growth to be quantified and compared in the context of the positional information thought to control them.
Keywords: A. thaliana; convolutional neural networks; developmental biology; morphogenesis; plant biology; positional information; quantification; segmentation.
© 2022, Strauss et al.
Conflict of interest statement
SS, AR, BL, DE, NB, NT, AR, SY, SR, AV, RT, MM, EE, CL, HB, MA, KS, GB, DK, JS, MT, RS No competing interests declared
Figures
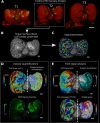
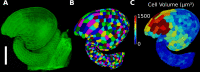
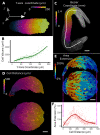
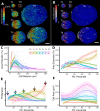
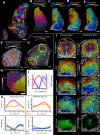
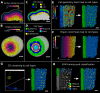
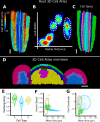
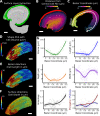
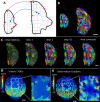
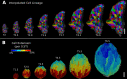
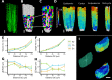
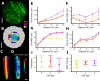
Similar articles
-
MorphoGraphX: A platform for quantifying morphogenesis in 4D.Elife. 2015 May 6;4:05864. doi: 10.7554/eLife.05864. Elife. 2015. PMID: 25946108 Free PMC article.
-
Positional Information and Pattern Formation.Curr Top Dev Biol. 2016;117:597-608. doi: 10.1016/bs.ctdb.2015.11.008. Epub 2016 Feb 1. Curr Top Dev Biol. 2016. PMID: 26970003 Review.
-
Positional information, positional error, and readout precision in morphogenesis: a mathematical framework.Genetics. 2015 Jan;199(1):39-59. doi: 10.1534/genetics.114.171850. Epub 2014 Oct 31. Genetics. 2015. PMID: 25361898 Free PMC article.
-
Accuracy of positional information provided by multiple morphogen gradients with correlated noise.Phys Rev E Stat Nonlin Soft Matter Phys. 2009 Jun;79(6 Pt 1):061905. doi: 10.1103/PhysRevE.79.061905. Epub 2009 Jun 4. Phys Rev E Stat Nonlin Soft Matter Phys. 2009. PMID: 19658522
-
Interplay between morphogen-directed positional information systems and physiological signaling.Dev Dyn. 2020 Mar;249(3):328-341. doi: 10.1002/dvdy.140. Epub 2019 Dec 20. Dev Dyn. 2020. PMID: 31794137 Free PMC article. Review.
Cited by
-
Tradeoff between speed and robustness in primordium initiation mediated by auxin-CUC1 interaction.Nat Commun. 2024 Jul 13;15(1):5911. doi: 10.1038/s41467-024-50172-9. Nat Commun. 2024. PMID: 39003301 Free PMC article.
-
A deep learning-based toolkit for 3D nuclei segmentation and quantitative analysis in cellular and tissue context.Development. 2024 Jul 15;151(14):dev202800. doi: 10.1242/dev.202800. Epub 2024 Jul 18. Development. 2024. PMID: 39036998 Free PMC article.
-
Growth directions and stiffness across cell layers determine whether tissues stay smooth or buckle.bioRxiv [Preprint]. 2025 Apr 2:2023.07.22.549953. doi: 10.1101/2023.07.22.549953. bioRxiv. 2025. PMID: 37546730 Free PMC article. Preprint.
-
CLAVATA signalling shapes barley inflorescence by controlling activity and determinacy of shoot meristem and rachilla.Nat Commun. 2025 Apr 26;16(1):3937. doi: 10.1038/s41467-025-59330-z. Nat Commun. 2025. PMID: 40287461 Free PMC article.
-
A future in 3D: Analyzing morphology in all dimensions.Plant Physiol. 2022 Jun 27;189(3):1175-1176. doi: 10.1093/plphys/kiac190. Plant Physiol. 2022. PMID: 35478044 Free PMC article. No abstract available.
References
-
- Barbier de Reuille P, Routier-Kierzkowska AL, Kierzkowski D, Bassel GW, Schüpbach T, Tauriello G, Bajpai N, Strauss S, Weber A, Kiss A, Burian A, Hofhuis H, Sapala A, Lipowczan M, Heimlicher MB, Robinson S, Bayer EM, Basler K, Koumoutsakos P, Roeder AHK, Aegerter-Wilmsen T, Nakayama N, Tsiantis M, Hay A, Kwiatkowska D, Xenarios I, Kuhlemeier C, Smith RS. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife. 2015;4:e05864. doi: 10.7554/eLife.05864. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

