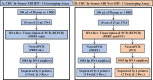
A Partially Multiplexed HIV Drug Resistance (HIVDR) Assay for Monitoring HIVDR Mutations of the Protease, Reverse-Transcriptase (PRRT), and Integrase (INT)
- PMID: 35510849
- PMCID: PMC9241735
- DOI: 10.1128/spectrum.01776-21
A Partially Multiplexed HIV Drug Resistance (HIVDR) Assay for Monitoring HIVDR Mutations of the Protease, Reverse-Transcriptase (PRRT), and Integrase (INT)
Abstract
As dolutegravir (DTG)-containing HIV regimens are scaled up globally, monitoring for HIV drug resistance (HIVDR) will become increasingly important. We designed a partially multiplexed HIVDR assay using Sanger sequencing technology to monitor HIVDR mutations in the protease, reverse-transcriptase (PRRT), and integrase (INT). A total of 213 clinical and analytical plasma and dried blood spot (DBS) samples were used in the evaluation. The assay detected a wide range of known HIV-1 subtypes and circulating recombinant forms (CRFs) of group M from 139 samples. INT accuracy showed that the average nucleotide (nt) sequence concordance was 99.8% for 75 plasma samples and 99.5% for 11 DBS samples compared with the reference sequences. The PRRT accuracy also demonstrated the average nucleotide sequence concordance was 99.5% for 57 plasma samples and 99.2% for 33 DBS samples. The major PRRT and INT DR mutations of all samples tested were concordant with those of the reference sequences using the Stanford HIV database (db). Amplification sensitivity of samples with viral load (VL) >5000 copies/mL showed plasma exceeded 95% of positivity, and DBS exceeded 90% for PRRT and INT. Samples with VL (1000 to 5000 copies/mL) showed plasma exceeded 90%, and DBS reached 88% positivity for PRRT and INT. Assay precision and reproducibility showed >99% nucleotide sequence concordance in each set of replicates for PRRT and INT. In conclusion, this HIVDR assay met WHO HIVDR assay performance criteria for surveillance, worked for plasma and DBS, used minimal sample volume, was sensitive, and was a potentially cost-effective tool to monitor HIVDR mutations in PRRT and INT. IMPORTANCE This HIVDR genotyping assay works for both plasma and DBS samples, requires low sample input, and is sensitive. This assay has the potential to be a user-friendly and cost-effective HIVDR assay because of its partially multiplexed design. Application of this genotyping assay will help HIVDR monitoring in HIV high-burdened countries using a DGT-based HIV drug regimen recommended by the U.S. President's Emergency Plan for AIDS Relief and the WHO.
Keywords: drug resistance; human immunodeficiency virus; integrase.
Conflict of interest statement
The authors declare a conflict of interest. Joshua DeVos is a co-inventor in U.S. patent US10053741B2. Robert Shafer has received research funding from Vela Diagnostics and InSilixa Inc.
Figures
Similar articles
-
High viral suppression and detection of dolutegravir-resistance associated mutations in treatment-experienced Tanzanian adults living with HIV-1 in Dar es Salaam.Sci Rep. 2023 Nov 22;13(1):20493. doi: 10.1038/s41598-023-47795-1. Sci Rep. 2023. PMID: 37993493 Free PMC article.
-
Affordable drug resistance genotyping of HIV-1 reverse transcriptase, protease and integrase genes, for resource limited settings.AIDS Res Ther. 2023 Feb 9;20(1):9. doi: 10.1186/s12981-023-00505-3. AIDS Res Ther. 2023. PMID: 36759801 Free PMC article.
-
Evaluation of an affordable HIV-1 virological failure assay and antiretroviral drug resistance genotyping protocol.J Virol Methods. 2013 Dec;194(1-2):300-7. doi: 10.1016/j.jviromet.2013.08.015. Epub 2013 Aug 28. J Virol Methods. 2013. PMID: 23994150
-
Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance.AIDS Rev. 2010 Oct-Dec;12(4):195-208. AIDS Rev. 2010. PMID: 21179184 Review.
-
The utility of integrating nanopore sequencing into routine HIV-1 drug resistance surveillance.Microb Genom. 2025 Mar;11(3):001375. doi: 10.1099/mgen.0.001375. Microb Genom. 2025. PMID: 40111248 Free PMC article. Review.
References
-
- UNAIDS. 2014. 90–90-90 An ambitious treatment target to help end the AIDS epidemic. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
-
- UNAIDS. 2014. Fast-Track - Ending the AIDS epidemic by 2030. https://www.unaids.org/en/resources/documents/2014/JC2686_WAD2014report.
-
- UNAIDS. 2020. Global HIV & AIDS statistics - 2020 fact sheet. https://www.unaids.org/en/resources/fact-sheet.
-
- WHO. 2017. HIV drug resistance report 2017. https://www.who.int/publications/i/item/9789241512831.
-
- WHO. 2019. HIV drug resistance report 2019. https://www.who.int/publications/i/item/WHO-CDS-HIV-19.21.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous