SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death
- PMID: 35510852
- PMCID: PMC9241873
- DOI: 10.1128/spectrum.01091-22
SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death
Abstract
Accumulating evidence suggests that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes various neurological symptoms in patients with coronavirus disease 2019 (COVID-19). The most dominant immune cells in the brain are microglia. Yet, the relationship between neurological manifestations, neuroinflammation, and host immune response of microglia to SARS-CoV-2 has not been well characterized. Here, we reported that SARS-CoV-2 can directly infect human microglia, eliciting M1-like proinflammatory responses, followed by cytopathic effects. Specifically, SARS-CoV-2 infected human microglial clone 3 (HMC3), leading to inflammatory activation and cell death. RNA sequencing (RNA-seq) analysis also revealed that endoplasmic reticulum (ER) stress and immune responses were induced in the early, and apoptotic processes in the late phases of viral infection. SARS-CoV-2-infected HMC3 showed the M1 phenotype and produced proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNF-α), but not the anti-inflammatory cytokine IL-10. After this proinflammatory activation, SARS-CoV-2 infection promoted both intrinsic and extrinsic death receptor-mediated apoptosis in HMC3. Using K18-hACE2 transgenic mice, murine microglia were also infected by intranasal inoculation of SARS-CoV-2. This infection induced the acute production of proinflammatory microglial IL-6 and TNF-α and provoked a chronic loss of microglia. Our findings suggest that microglia are potential mediators of SARS-CoV-2-induced neurological problems and, consequently, can be targets of therapeutic strategies against neurological diseases in patients with COVID-19. IMPORTANCE Recent studies reported neurological and cognitive sequelae in patients with COVID-19 months after the viral infection with several symptoms, including ageusia, anosmia, asthenia, headache, and brain fog. Our conclusions raise awareness of COVID-19-related microglia-mediated neurological disorders to develop treatment strategies for the affected patients. We also indicated that HMC3 was a novel human cell line susceptible to SARS-CoV-2 infection that exhibited cytopathic effects, which could be further used to investigate cellular and molecular mechanisms of neurological manifestations of patients with COVID-19.
Keywords: M1 polarization; SARS-CoV-2; apoptosis; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

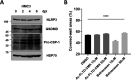
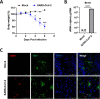

Similar articles
-
Microglia Do Not Restrict SARS-CoV-2 Replication following Infection of the Central Nervous System of K18-Human ACE2 Transgenic Mice.J Virol. 2022 Feb 23;96(4):e0196921. doi: 10.1128/jvi.01969-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935438 Free PMC article.
-
Microglia do not restrict SARS-CoV-2 replication following infection of the central nervous system of K18-hACE2 transgenic mice.bioRxiv [Preprint]. 2021 Nov 17:2021.11.15.468761. doi: 10.1101/2021.11.15.468761. bioRxiv. 2021. Update in: J Virol. 2022 Feb 23;96(4):e0196921. doi: 10.1128/jvi.01969-21. PMID: 34816260 Free PMC article. Updated. Preprint.
-
SARS-CoV-2 ORF3a induces COVID-19-associated kidney injury through HMGB1-mediated cytokine production.mBio. 2024 Nov 13;15(11):e0230824. doi: 10.1128/mbio.02308-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39345136 Free PMC article.
-
Microglial Inflammatory Responses to SARS-CoV-2 Infection: A Comprehensive Review.Cell Mol Neurobiol. 2023 Dec 15;44(1):2. doi: 10.1007/s10571-023-01444-3. Cell Mol Neurobiol. 2023. PMID: 38099973 Free PMC article. Review.
-
Severe Acute Respiratory Syndrome Coronavirus 2 Impact on the Central Nervous System: Are Astrocytes and Microglia Main Players or Merely Bystanders?ASN Neuro. 2020 Jan-Dec;12:1759091420954960. doi: 10.1177/1759091420954960. ASN Neuro. 2020. PMID: 32878468 Free PMC article. Review.
Cited by
-
Activation of NF-κB/MAPK signaling and induction of apoptosis by salicylate synthase NbtS in Nocardia farcinica promotes neuroinflammation development.mSystems. 2024 Oct 22;9(10):e0089324. doi: 10.1128/msystems.00893-24. Epub 2024 Sep 6. mSystems. 2024. PMID: 39240104 Free PMC article.
-
COVID-19 and neurodegeneration: The mitochondrial connection.Aging Cell. 2022 Nov;21(11):e13727. doi: 10.1111/acel.13727. Epub 2022 Oct 11. Aging Cell. 2022. PMID: 36219531 Free PMC article.
-
Non-Productive Infection of Glial Cells with SARS-CoV-2 in Hamster Organotypic Cerebellar Slice Cultures.Viruses. 2022 Jun 3;14(6):1218. doi: 10.3390/v14061218. Viruses. 2022. PMID: 35746689 Free PMC article.
-
Orchestration of antiviral responses within the infected central nervous system.Cell Mol Immunol. 2024 Sep;21(9):943-958. doi: 10.1038/s41423-024-01181-7. Epub 2024 Jul 12. Cell Mol Immunol. 2024. PMID: 38997413 Free PMC article. Review.
-
Revisiting the Pathogenesis of X-Linked Adrenoleukodystrophy.Genes (Basel). 2025 May 17;16(5):590. doi: 10.3390/genes16050590. Genes (Basel). 2025. PMID: 40428412 Free PMC article. Review.
References
-
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, Coles JP, Manji H, Al-Shahi Salman R, Menon DK, Nicholson TR, Benjamin LA, Carson A, Smith C, Turner MR, Solomon T, Kneen R, Pett SL, Galea I, Thomas RH, Michael BD, Allen C, Archibald N, Arkell J, Arthur-Farraj P, Baker M, Ball H, Bradley-Barker V, Brown Z, Bruno S, Carey L, Carswell C, Chakrabarti A, Choulerton J, Daher M, Davies R, Di Marco Barros R, Dima S, Dunley R, Dutta D, Ellis R, Everitt A, Fady J, Fearon P, Fisniku L, Gbinigie I, et al. . 2020. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7:875–882. doi:10.1016/S2215-0366(20)30287-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

