Transcription factors BBX11 and HY5 interdependently regulate the molecular and metabolic responses to UV-B
- PMID: 35511140
- PMCID: PMC9342961
- DOI: 10.1093/plphys/kiac195
Transcription factors BBX11 and HY5 interdependently regulate the molecular and metabolic responses to UV-B
Abstract
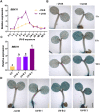
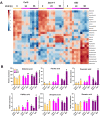
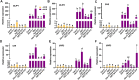
UV-B radiation acts as a developmental cue and a stress factor for plants, depending on dose. Activation of the transcription factor ELONGATED HYPOCOTYL 5 (HY5) in a UV RESISTANCE LOCUS 8 (UVR8)-dependent manner leads to the induction of a broad set of genes under UV-B. However, the underlying molecular mechanisms regulating this process are less understood. Here, we use molecular, biochemical, genetic, and metabolomic tools to identify the B-BOX transcription factor B-BOX PROTEIN 11 (BBX11) as a component of the molecular response to UV-B in Arabidopsis (Arabidopsis thaliana). BBX11 expression is induced by UV-B in a dose-dependent manner. Under low UV-B, BBX11 regulates hypocotyl growth suppression, whereas it protects plants exposed to high UV-B radiation by promoting the accumulation of photo-protective phenolics and antioxidants, and inducing DNA repair genes. Our genetic studies indicate that BBX11 regulates hypocotyl elongation under UV-B partially dependent on HY5. Overexpression of BBX11 can partially rescue the high UV-B sensitivity of hy5, suggesting that HY5-mediated UV-B stress tolerance is partially dependent on BBX11. HY5 regulates the UV-B-mediated induction of BBX11 by directly binding to its promoter. BBX11 reciprocally regulates the mRNA and protein levels of HY5. We report here the role of a BBX11-HY5 feedback loop in regulating photomorphogenesis and stress tolerance under UV-B.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





Comment in
-
B-BOXing against UV rays: The BBX11-HY5 feedback loop regulates plant ultraviolet B tolerance.Plant Physiol. 2022 Aug 1;189(4):1904-1905. doi: 10.1093/plphys/kiac248. Plant Physiol. 2022. PMID: 35639739 Free PMC article. No abstract available.
References
-
- Bai SL, Saito T, Honda C, Hatsuyama Y, Ito A, Moriguchi T (2014) An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta 240: 1051–1062 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

