Early and Rapid Identification of COVID-19 Patients with Neutralizing Type I Interferon Auto-antibodies
- PMID: 35511314
- PMCID: PMC9069123
- DOI: 10.1007/s10875-022-01252-2
Early and Rapid Identification of COVID-19 Patients with Neutralizing Type I Interferon Auto-antibodies
Abstract
Purpose: Six to 19% of critically ill COVID-19 patients display circulating auto-antibodies against type I interferons (IFN-AABs). Here, we establish a clinically applicable strategy for early identification of IFN-AAB-positive patients for potential subsequent clinical interventions.
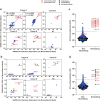
Methods: We analyzed sera of 430 COVID-19 patients from four hospitals for presence of IFN-AABs by ELISA. Binding specificity and neutralizing activity were evaluated via competition assay and virus-infection-based neutralization assay. We defined clinical parameters associated with IFN-AAB positivity. In a subgroup of critically ill patients, we analyzed effects of therapeutic plasma exchange (TPE) on the levels of IFN-AABs, SARS-CoV-2 antibodies and clinical outcome.
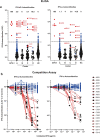
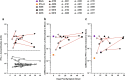
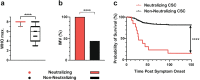
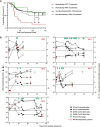
Results: The prevalence of neutralizing AABs to IFN-α and IFN-ω in COVID-19 patients from all cohorts was 4.2% (18/430), while being undetectable in an uninfected control cohort. Neutralizing IFN-AABs were detectable exclusively in critically affected (max. WHO score 6-8), predominantly male (83%) patients (7.6%, 18/237 for IFN-α-AABs and 4.6%, 11/237 for IFN-ω-AABs in 237 patients with critical COVID-19). IFN-AABs were present early post-symptom onset and at the peak of disease. Fever and oxygen requirement at hospital admission co-presented with neutralizing IFN-AAB positivity. IFN-AABs were associated with lower probability of survival (7.7% versus 80.9% in patients without IFN-AABs). TPE reduced levels of IFN-AABs in three of five patients and may increase survival of IFN-AAB-positive patients compared to those not undergoing TPE.
Conclusion: IFN-AABs may serve as early biomarker for the development of severe COVID-19. We propose to implement routine screening of hospitalized COVID-19 patients for rapid identification of patients with IFN-AABs who most likely benefit from specific therapies.
Keywords: Autoantibodies; COVID-19; SARS-CoV-2; Type I interferon.
© 2022. The Author(s).
Conflict of interest statement
V.M.C is named together with Euroimmun GmbH on a patent application filed recently regarding the diagnostic of SARS-CoV-2 by antibody testing. Technische Universität Berlin, Freie Universität Berlin, and Charité—Universitätsmedizin have filed a patent application for siRNAs inhibiting SARS-CoV-2 replication with D.N. as co-author. J.C.S, T.S. (full departmental disclosure): the department of Intensive Care Medicine has/had research and/or development/consulting contracts with (full disclosure) Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH/SA, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Phagenesis Ltd, Cytel, and Nestlé. No personal financial gains resulted from respective development/consulting contracts and/or educational grants.
Figures


Similar articles
-
Autoantibodies against type I interferons in COVID-19 infection: A systematic review and meta-analysis.Int J Infect Dis. 2023 May;130:147-152. doi: 10.1016/j.ijid.2023.03.011. Epub 2023 Mar 11. Int J Infect Dis. 2023. PMID: 36907547 Free PMC article.
-
Salivary autoantibodies to type I IFNs: Mirror plasma levels, predispose to severe COVID-19, and enhance feasibility for clinical screening.Heart Lung. 2024 Jun-Aug;66:31-36. doi: 10.1016/j.hrtlng.2024.03.005. Epub 2024 Mar 27. Heart Lung. 2024. PMID: 38547583
-
Autoantibodies Neutralizing Type III Interferons Are Uncommon in Patients with Severe Coronavirus Disease 2019 Pneumonia.J Interferon Cytokine Res. 2023 Sep;43(9):379-393. doi: 10.1089/jir.2023.0003. Epub 2023 May 29. J Interferon Cytokine Res. 2023. PMID: 37253131 Free PMC article.
-
Neutralizing Type I Interferon Autoantibodies in Japanese Patients with Severe COVID-19.J Clin Immunol. 2022 Oct;42(7):1360-1370. doi: 10.1007/s10875-022-01308-3. Epub 2022 Jun 29. J Clin Immunol. 2022. PMID: 35764767 Free PMC article.
-
Circulating Type I Interferon Levels and COVID-19 Severity: A Systematic Review and Meta-Analysis.Front Immunol. 2021 May 12;12:657363. doi: 10.3389/fimmu.2021.657363. eCollection 2021. Front Immunol. 2021. PMID: 34054820 Free PMC article.
Cited by
-
Autoantibodies against type I interferons in COVID-19 infection: A systematic review and meta-analysis.Int J Infect Dis. 2023 May;130:147-152. doi: 10.1016/j.ijid.2023.03.011. Epub 2023 Mar 11. Int J Infect Dis. 2023. PMID: 36907547 Free PMC article.
-
From rare disorders of immunity to common determinants of infection: Following the mechanistic thread.Cell. 2022 Aug 18;185(17):3086-3103. doi: 10.1016/j.cell.2022.07.004. Cell. 2022. PMID: 35985287 Free PMC article. Review.
-
Autoantibodies Neutralizing Type I IFNs in the Bronchoalveolar Lavage of at Least 10% of Patients During Life-Threatening COVID-19 Pneumonia.J Clin Immunol. 2023 Aug;43(6):1093-1103. doi: 10.1007/s10875-023-01512-9. Epub 2023 May 20. J Clin Immunol. 2023. PMID: 37209324 Free PMC article.
-
Human genetic and immunological determinants of SARS-CoV-2 infection and multisystem inflammatory syndrome in children.Clin Exp Immunol. 2025 Jan 21;219(1):uxae062. doi: 10.1093/cei/uxae062. Clin Exp Immunol. 2025. PMID: 39028583 Free PMC article. Review.
-
A sensitive assay for measuring whole-blood responses to type I IFNs.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2402983121. doi: 10.1073/pnas.2402983121. Epub 2024 Sep 23. Proc Natl Acad Sci U S A. 2024. PMID: 39312669 Free PMC article.
References
-
- Pfortmueller CA, Spinetti T, Urman RD, Luedi MM, Schefold JC. COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment–a narrative review. Best Pract Res Clin Anaesthesiol. 2021;35(3):351–368. doi: 10.1016/j.bpa.2020.12.011. - DOI - PMC - PubMed
-
- Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, et al. Risk stratification of patients admitted to hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

