Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter
- PMID: 35511454
- PMCID: PMC9629431
- DOI: 10.1093/neuonc/noac116
Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter
Abstract
Background: Nearly all patients with newly diagnosed glioblastoma experience recurrence following standard-of-care radiotherapy (RT) + temozolomide (TMZ). The purpose of the phase III randomized CheckMate 548 study was to evaluate RT + TMZ combined with the immune checkpoint inhibitor nivolumab (NIVO) or placebo (PBO) in patients with newly diagnosed glioblastoma with methylated MGMT promoter (NCT02667587).
Methods: Patients (N = 716) were randomized 1:1 to NIVO [(240 mg every 2 weeks × 8, then 480 mg every 4 weeks) + RT (60 Gy over 6 weeks) + TMZ (75 mg/m2 once daily during RT, then 150-200 mg/m2 once daily on days 1-5 of every 28-day cycle × 6)] or PBO + RT + TMZ following the same regimen. The primary endpoints were progression-free survival (PFS) and overall survival (OS) in patients without baseline corticosteroids and in all randomized patients.
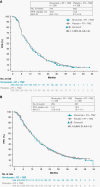
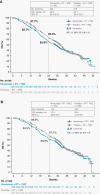
Results: As of December 22, 2020, median (m)PFS (blinded independent central review) was 10.6 months (95% CI, 8.9-11.8) with NIVO + RT + TMZ vs 10.3 months (95% CI, 9.7-12.5) with PBO + RT + TMZ (HR, 1.1; 95% CI, 0.9-1.3) and mOS was 28.9 months (95% CI, 24.4-31.6) vs 32.1 months (95% CI, 29.4-33.8), respectively (HR, 1.1; 95% CI, 0.9-1.3). In patients without baseline corticosteroids, mOS was 31.3 months (95% CI, 28.6-34.8) with NIVO + RT + TMZ vs 33.0 months (95% CI, 31.0-35.1) with PBO + RT + TMZ (HR, 1.1; 95% CI, 0.9-1.4). Grade 3/4 treatment-related adverse event rates were 52.4% vs 33.6%, respectively.
Conclusions: NIVO added to RT + TMZ did not improve survival in patients with newly diagnosed glioblastoma with methylated or indeterminate MGMT promoter. No new safety signals were observed.
Keywords: MGMT promoter; PD-L1; glioblastoma; nivolumab; temozolomide.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- TEMODAR (temozolomide) US Prescribing Information: Merck Sharp & Dohme Corp, November 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

