Relative Vaccine Effectiveness of a Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine Booster Dose Against the Omicron Variant
- PMID: 35511586
- PMCID: PMC11487159
- DOI: 10.1093/cid/ciac328
Relative Vaccine Effectiveness of a Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine Booster Dose Against the Omicron Variant
Abstract
Background: The current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines may be less effective against the Omicron variant than against earlier variants. With recent resurgence of SARS-CoV-2 cases, the role of booster doses of the vaccine needs to be highlighted.
Methods: Using a retrospective cohort study design emulating a target trial, we determined the relative vaccine effectiveness (RVE) of a homologous booster dose of a SARS-CoV-2 messenger RNA (mRNA) vaccine compared with the primary vaccine series alone in preventing infection, hospitalization, and intensive care unit admission, and death in the Department of Veterans Affairs healthcare system in the United States. Among infection-free survivors who received 2 doses of a mRNA vaccine before 30 April 2021, we identified those who received a booster between 22 September and 25 December 2021 and matched them 1:1 with individuals who did not receive a booster.
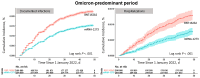
Results: Among 2 384 272 previously uninfected persons with 2 doses of an mRNA vaccine by 30 April 2021, we identified 462 950 booster recipients between 22 September and 25 December 2021, who were matched 1:1 with non-booster recipients. The RVE (95% confidence interval) was 19% (17%-22%) for confirmed infection, 52% (46%-57%) for hospitalization, and 83% (65%-92%) for intensive care unit admission or death. Recipients of the mRNA-1273 vaccine had a lower cumulative incidence of infections and hospitalizations than recipients of the BNT162b2 vaccine (log-rank P <.001 for both comparisons).
Conclusions: While the RVE of SARS-CoV-2 mRNA booster vaccine dose in preventing infection against the Omicron variant is low, it is substantial in preventing hospitalization and high in preventing the most severe/critical disease.
Keywords: Delta variant; Omicron variant; SARS-CoV-2; booster; vaccine effectiveness.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2022.
Conflict of interest statement
Potential conflicts of interest. A. A. B. has received investigator-initiated grant funding from Gilead Sciences (to the institution, Veterans Health Foundation of Pittsburgh), unrelated to the work presented here. F. B. M. is supported by a grant from the National Institutes of Health (career development award K23GM132688), related to this work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
When Emulating a Trial, Do as the Trialists Do: Missteps in Estimating Relative Effectiveness of a Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Booster Dose.Clin Infect Dis. 2023 Jan 6;76(1):176-177. doi: 10.1093/cid/ciac700. Clin Infect Dis. 2023. PMID: 36041013 Free PMC article. No abstract available.
References
-
- Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021; 27:1614–21. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

