Organization and engagement of a prefrontal-olfactory network during olfactory selective attention
- PMID: 35511680
- PMCID: PMC9930634
- DOI: 10.1093/cercor/bhac153
Organization and engagement of a prefrontal-olfactory network during olfactory selective attention
Abstract
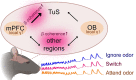
Background: Sensory perception is profoundly shaped by attention. Attending to an odor strongly regulates if and how it is perceived - yet the brain systems involved in this process are unknown. Here we report integration of the medial prefrontal cortex (mPFC), a collection of brain regions integral to attention, with the olfactory system in the context of selective attention to odors.
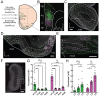
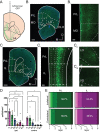
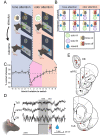
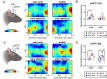
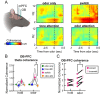
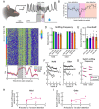
Methods: First, we used tracing methods to establish the tubular striatum (TuS, also known as the olfactory tubercle) as the primary olfactory region to receive direct mPFC input in rats. Next, we recorded (i) local field potentials from the olfactory bulb (OB), mPFC, and TuS, or (ii) sniffing, while rats completed an olfactory selective attention task.
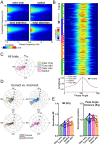
Results: Gamma power and coupling of gamma oscillations with theta phase were consistently high as rats flexibly switched their attention to odors. Beta and theta synchrony between mPFC and olfactory regions were elevated as rats switched their attention to odors. Finally, we found that sniffing was consistent despite shifting attentional demands, suggesting that the mPFC-OB theta coherence is independent of changes in active sampling.
Conclusions: Together, these findings begin to define an olfactory attention network wherein mPFC activity, as well as that within olfactory regions, are coordinated based upon attentional states.
Keywords: active sampling; neural circuits; neural oscillations; olfactory cortex; prefrontal cortex; sniffing.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
The olfactory bulb coordinates the ventral hippocampus-medial prefrontal cortex circuit during spatial working memory performance.J Physiol Sci. 2022 Apr 25;72(1):9. doi: 10.1186/s12576-022-00833-5. J Physiol Sci. 2022. PMID: 35468718 Free PMC article.
-
Gamma and Beta Oscillations Define a Sequence of Neurocognitive Modes Present in Odor Processing.J Neurosci. 2016 Jul 20;36(29):7750-67. doi: 10.1523/JNEUROSCI.0569-16.2016. J Neurosci. 2016. PMID: 27445151 Free PMC article.
-
Olfactory bulb-medial prefrontal cortex theta synchronization is associated with anxiety.Sci Rep. 2024 May 27;14(1):12101. doi: 10.1038/s41598-024-63101-z. Sci Rep. 2024. PMID: 38802558 Free PMC article.
-
The sniff as a unit of olfactory processing.Chem Senses. 2006 Feb;31(2):167-79. doi: 10.1093/chemse/bjj016. Epub 2005 Dec 8. Chem Senses. 2006. PMID: 16339265 Review.
-
Circuit oscillations in odor perception and memory.Prog Brain Res. 2014;208:223-51. doi: 10.1016/B978-0-444-63350-7.00009-7. Prog Brain Res. 2014. PMID: 24767485 Review.
Cited by
-
Respiratory Coupled Oscillations as a Mechanism of Attention to the Olfactory Environment.J Neurosci. 2024 Feb 21;44(8):e1866232024. doi: 10.1523/JNEUROSCI.1866-23.2024. J Neurosci. 2024. PMID: 38383486 Free PMC article. No abstract available.
-
Identification of the Neural Correlates Underlying Conflict Resolution Performance Using a Rodent Analogue of the Stroop Tests.Neuroscience. 2023 Aug 1;524:79-88. doi: 10.1016/j.neuroscience.2023.05.024. Epub 2023 Jun 7. Neuroscience. 2023. PMID: 37290682 Free PMC article.
-
Fast updating feedback from piriform cortex to the olfactory bulb relays multimodal reward contingency signals during rule-reversal.bioRxiv [Preprint]. 2023 Sep 13:2023.09.12.557267. doi: 10.1101/2023.09.12.557267. bioRxiv. 2023. Update in: Nat Commun. 2025 Jan 22;16(1):937. doi: 10.1038/s41467-025-56023-5. PMID: 37745564 Free PMC article. Updated. Preprint.
-
The roles of rat medial prefrontal and orbitofrontal cortices in relapse to cocaine-seeking: A comparison across methods for identifying neurocircuits.Addict Neurosci. 2022 Dec;4:100031. doi: 10.1016/j.addicn.2022.100031. Epub 2022 Jul 28. Addict Neurosci. 2022. PMID: 36277334 Free PMC article.
-
Neuroglia's signaling: orchestrating neuronal gamma synchrony?Curr Opin Neurol. 2025 Aug 1;38(4):298-305. doi: 10.1097/WCO.0000000000001392. Epub 2025 Jun 5. Curr Opin Neurol. 2025. PMID: 40471684 Review.
References
-
- Ährlund-Richter S, Xuan Y, van Lunteren JA, Kim H, Ortiz C, Pollak Dorocic I, Meletis K, Carlén M. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat Neurosci. 2019:22:657–668. - PubMed
-
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003:18:2059–2068. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

