Effectiveness of a COVID-19 Additional Primary or Booster Vaccine Dose in Preventing SARS-CoV-2 Infection Among Nursing Home Residents During Widespread Circulation of the Omicron Variant - United States, February 14-March 27, 2022
- PMID: 35511708
- PMCID: PMC9098239
- DOI: 10.15585/mmwr.mm7118a4
Effectiveness of a COVID-19 Additional Primary or Booster Vaccine Dose in Preventing SARS-CoV-2 Infection Among Nursing Home Residents During Widespread Circulation of the Omicron Variant - United States, February 14-March 27, 2022
Abstract
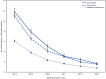
Nursing home residents have experienced disproportionally high levels of COVID-19-associated morbidity and mortality and were prioritized for early COVID-19 vaccination (1). Following reported declines in vaccine-induced immunity after primary series vaccination, defined as receipt of 2 primary doses of an mRNA vaccine (BNT162b2 [Pfizer-BioNTech] or mRNA-1273 [Moderna]) or 1 primary dose of Ad26.COV2 (Johnson & Johnson [Janssen]) vaccine (2), CDC recommended that all persons aged ≥12 years receive a COVID-19 booster vaccine dose.* Moderately to severely immunocompromised persons, a group that includes many nursing home residents, are also recommended to receive an additional primary COVID-19 vaccine dose.† Data on vaccine effectiveness (VE) of an additional primary or booster dose against infection with SARS-CoV-2 (the virus that causes COVID-19) among nursing home residents are limited, especially against the highly transmissible B.1.1.529 and BA.2 (Omicron) variants. Weekly COVID-19 surveillance and vaccination coverage data among nursing home residents, reported by skilled nursing facilities (SNFs) to CDC's National Healthcare Safety Network (NHSN)§ during February 14-March 27, 2022, when the Omicron variant accounted for >99% of sequenced isolates, were analyzed to estimate relative VE against infection for any COVID-19 additional primary or booster dose compared with primary series vaccination. After adjusting for calendar week and variability across SNFs, relative VE of a COVID-19 additional primary or booster dose was 46.9% (95% CI = 44.8%-48.9%). These findings indicate that among nursing home residents, COVID-19 additional primary or booster doses provide greater protection against Omicron variant infection than does primary series vaccination alone. All immunocompromised nursing home residents should receive an additional primary dose, and all nursing home residents should receive a booster dose, when eligible, to protect against COVID-19. Efforts to keep nursing home residents up to date with vaccination should be implemented in conjunction with other COVID-19 prevention strategies, including testing and vaccination of nursing home staff members and visitors.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163–6. 10.15585/mmwr.mm7034e3 - DOI - PMC - PubMed
-
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255–63. 10.15585/mmwr.mm7107e2 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

