Decay rate of antiS1/S2 IgG serum levels after 6 months of BNT162b2 vaccination in a cohort of COVID-19-naive and COVID-19-experienced subjects
- PMID: 35511791
- PMCID: PMC9897653
- DOI: 10.1080/21645515.2022.2060018
Decay rate of antiS1/S2 IgG serum levels after 6 months of BNT162b2 vaccination in a cohort of COVID-19-naive and COVID-19-experienced subjects
Abstract
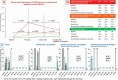
Vaccination toward SARS-CoV-2 reduced mortality and 'boosters' are being implemented. We offer scientific contribution about IgG production in the COVID-19 experienced population. From January 2021 to March 2021, 183 residents and staff from the Elderly Nursing Home "San Giuseppe Moscati" who had received two doses of the BNT162b2 vaccine were enrolled. The antibody response was assessed by the DiaSorin LIAISON-CLIA S1/S2® IgG solution. Cutoff levels for response (>39 BAU/mL) and neutralizing activity (>208 BAU/mL) were derived from DiaSorin official data. Serology was assessed before and after the first vaccination, and 2 weeks and 6 months after the second vaccination. Anti-S IgG in COVID-19 experienced, baseline IgG producers spiked after the first vaccination to median 5044 BAU/mL and decayed at 6 months to 2467.4 BAU/mL. Anti-S IgG in COVID-19 experienced, baseline IgG non-producers spiked after the second vaccination to median 1701.7 BAU/mL and decayed at 6 months to 904.8 BAU/mL. Anti-S IgG in COVID-19 naïve subjects spiked after the second vaccination to median 546 BAU/mL and decayed at 6 months to 319.8 BAU/mL. The differences between sequential timepoint levels in each group were statistically significant (p < .0001). Serology analysis revealed different kinetics between COVID-19 experienced subjects depending on baseline response, possibly predicting different IgG persistence in blood.
Keywords: COVID-19; IgG; SARS-CoV-2; antibody; decline; kinetics; spike; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- COVID-19 vaccine market dashboard. [accessed 2022 Feb 4]. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
-
- Hopkins’ J COVID-19 Dashboard. [accessed 2022 Feb 4]. https://coronavirus.jhu.edu/map.html
-
- Niesen MJM, Anand P, Silvert E, Niesen MJM, Corchado-Garcia J, O’Horo JC, Virk A, Swift MD, Halamka J, Badley AD, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. MedRxiv. 2021. 2021.07.01.21259833. doi:10.1101/2021.07.01.21259833. - DOI
-
- European Medicines Agency . COVID-19 Vaccines [accessed 2022 Mar 12]. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr...
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–5. doi:10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
