Intestine-to-neuronal signaling alters risk-taking behaviors in food-deprived Caenorhabditis elegans
- PMID: 35511794
- PMCID: PMC9070953
- DOI: 10.1371/journal.pgen.1010178
Intestine-to-neuronal signaling alters risk-taking behaviors in food-deprived Caenorhabditis elegans
Abstract
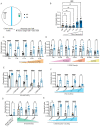
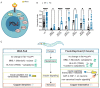
Animals integrate changes in external and internal environments to generate behavior. While neural circuits detecting external cues have been mapped, less is known about how internal states like hunger are integrated into behavioral outputs. Here, we use the nematode C. elegans to examine how changes in internal nutritional status affect chemosensory behaviors. We show that acute food deprivation leads to a reversible decline in repellent, but not attractant, sensitivity. This behavioral change requires two conserved transcription factors MML-1 (MondoA) and HLH-30 (TFEB), both of which translocate from the intestinal nuclei to the cytoplasm during food deprivation. Next, we identify the insulin-like peptide INS-31 as a candidate ligand relaying food-status signals from the intestine to other tissues. Further, we show that neurons likely use the DAF-2 insulin receptor and AGE-1/PI-3 Kinase, but not DAF-16/FOXO to integrate these intestine-released peptides. Altogether, our study shows how internal food status signals are integrated by transcription factors and intestine-neuron signaling to generate flexible behaviors via the gut-brain axis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Dugatkin LA. Principles of animal behavior: University of Chicago Press; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

