Exploring Motor Neuron Diseases Using iPSC Platforms
- PMID: 35511862
- PMCID: PMC9199844
- DOI: 10.1093/stmcls/sxab006
Exploring Motor Neuron Diseases Using iPSC Platforms
Abstract
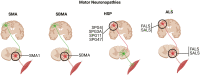
The degeneration of motor neurons is a pathological hallmark of motor neuron diseases (MNDs), but emerging evidence suggests that neuronal vulnerability extends well beyond this cell subtype. The ability to assess motor function in the clinic is limited to physical examination, electrophysiological measures, and tissue-based or neuroimaging techniques which lack the resolution to accurately assess neuronal dysfunction as the disease progresses. Spinal muscular atrophy (SMA), spinal and bulbar muscular atrophy (SBMA), hereditary spastic paraplegia (HSP), and amyotrophic lateral sclerosis (ALS) are all MNDs with devastating clinical outcomes that contribute significantly to disease burden as patients are no longer able to carry out normal activities of daily living. The critical need to accurately assess the cause and progression of motor neuron dysfunction, especially in the early stages of those diseases, has motivated the use of human iPSC-derived motor neurons (hiPSC-MN) to study the neurobiological mechanisms underlying disease pathogenesis and to generate platforms for therapeutic discovery and testing. As our understanding of MNDs has grown, so too has our need to develop more complex in vitro models which include hiPSC-MN co-cultured with relevant non-neuronal cells in 2D as well as in 3D organoid and spheroid systems. These more complex hiPSC-derived culture systems have led to the implementation of new technologies, including microfluidics, multielectrode array, and machine learning which offer novel insights into the functional correlates of these emerging model systems.
Keywords: ALS; hereditary spastic paraplegia; motor neuron disease; spinal and bulbar muscular atrophy; spinal muscular atrophy; stem cell.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1-13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

