ATP synthesis in an ancient ATP synthase at low driving forces
- PMID: 35512103
- PMCID: PMC9171764
- DOI: 10.1073/pnas.2201921119
ATP synthesis in an ancient ATP synthase at low driving forces
Abstract
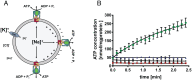
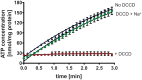
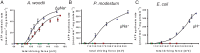
Hyperthermophilic archaea are close to the origin of life. Some hyperthermophilic anaerobic archaea live under strong energy limitation and have to make a living near thermodynamic equilibrium. Obviously, this requires adaptations of the energy-conserving machinery to harness small energy increments. Their ATP synthases often have an unusual motor subunit c that is predicted to prevent ATP synthesis. We have purified and reconstituted into liposomes such an archaeal ATP synthase found in a mesophilic bacterium. The enzyme indeed synthesized ATP at physiological membrane potentials, despite its unusual c subunit, but the minimal driving force for ATP synthesis was found to be even lower than in ATP synthases with usual c subunits. These data not only reveal an intermediate in the transition from ATP hydrolases to ATP synthases but also give a rationale for a bioenergetic adaptation of microbial growth near the thermodynamic equilibrium.
Keywords: ATP synthesis; acetogenic bacteria; archaea; bioenergetics; driving forces.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Senior A. E., ATP synthesis by oxidative phosphorylation. Physiol. Rev. 68, 177–231 (1988). - PubMed
-
- Kühlbrandt W., Davies K. M., Rotary ATPases: A new twist to an ancient machine. Trends Biochem. Sci. 41, 106–116 (2016). - PubMed
-
- Walker J. E., The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16 (2013). - PubMed
-
- Grüber G., Wieczorek H., Harvey W. R., Müller V., Structure-function relationships of A-, F- and V-ATPases. J. Exp. Biol. 204, 2597–2605 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

