Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord
- PMID: 35512359
- PMCID: PMC9473357
- DOI: 10.1093/brain/awac165
Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord
Abstract
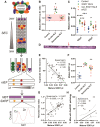
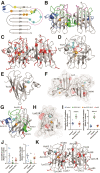
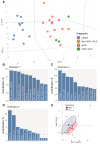
Aberrant self-assembly and toxicity of wild-type and mutant superoxide dismutase 1 (SOD1) has been widely examined in silico, in vitro and in transgenic animal models of amyotrophic lateral sclerosis. Detailed examination of the protein in disease-affected tissues from amyotrophic lateral sclerosis patients, however, remains scarce. We used histological, biochemical and analytical techniques to profile alterations to SOD1 protein deposition, subcellular localization, maturation and post-translational modification in post-mortem spinal cord tissues from amyotrophic lateral sclerosis cases and controls. Tissues were dissected into ventral and dorsal spinal cord grey matter to assess the specificity of alterations within regions of motor neuron degeneration. We provide evidence of the mislocalization and accumulation of structurally disordered, immature SOD1 protein conformers in spinal cord motor neurons of SOD1-linked and non-SOD1-linked familial amyotrophic lateral sclerosis cases, and sporadic amyotrophic lateral sclerosis cases, compared with control motor neurons. These changes were collectively associated with instability and mismetallation of enzymatically active SOD1 dimers, as well as alterations to SOD1 post-translational modifications and molecular chaperones governing SOD1 maturation. Atypical changes to SOD1 protein were largely restricted to regions of neurodegeneration in amyotrophic lateral sclerosis cases, and clearly differentiated all forms of amyotrophic lateral sclerosis from controls. Substantial heterogeneity in the presence of these changes was also observed between amyotrophic lateral sclerosis cases. Our data demonstrate that varying forms of SOD1 proteinopathy are a common feature of all forms of amyotrophic lateral sclerosis, and support the presence of one or more convergent biochemical pathways leading to SOD1 proteinopathy in amyotrophic lateral sclerosis. Most of these alterations are specific to regions of neurodegeneration, and may therefore constitute valid targets for therapeutic development.
Keywords: amyotrophic lateral sclerosis; mislocalization; neurodegeneration; post-translational modifications; superoxide dismutase-1.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Rosen DR, Siddique T, Patterson D, et al. . Mutations in Cu/Zn superoxide-dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature.1993;362:59–62. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

