N-dependent dynamics of root growth and nitrate and ammonium uptake are altered by the bacterium Herbaspirillum seropedicae in the cereal model Brachypodium distachyon
- PMID: 35512445
- PMCID: PMC9440436
- DOI: 10.1093/jxb/erac184
N-dependent dynamics of root growth and nitrate and ammonium uptake are altered by the bacterium Herbaspirillum seropedicae in the cereal model Brachypodium distachyon
Abstract
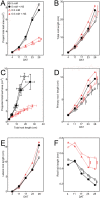
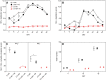
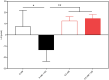
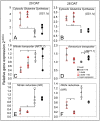
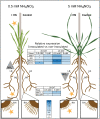
Nitrogen (N) fixation in cereals by root-associated bacteria is a promising solution for reducing use of chemical N fertilizers in agriculture. However, plant and bacterial responses are unpredictable across environments. We hypothesized that cereal responses to N-fixing bacteria are dynamic, depending on N supply and time. To quantify the dynamics, a gnotobiotic, fabricated ecosystem (EcoFAB) was adapted to analyse N mass balance, to image shoot and root growth, and to measure gene expression of Brachypodium distachyon inoculated with the N-fixing bacterium Herbaspirillum seropedicae. Phenotyping throughput of EcoFAB-N was 25-30 plants h-1 with open software and imaging systems. Herbaspirillum seropedicae inoculation of B. distachyon shifted root and shoot growth, nitrate versus ammonium uptake, and gene expression with time; directions and magnitude depended on N availability. Primary roots were longer and root hairs shorter regardless of N, with stronger changes at low N. At higher N, H. seropedicae provided 11% of the total plant N that came from sources other than the seed or the nutrient solution. The time-resolved phenotypic and molecular data point to distinct modes of action: at 5 mM NH4NO3 the benefit appears through N fixation, while at 0.5 mM NH4NO3 the mechanism appears to be plant physiological, with H. seropedicae promoting uptake of N from the root medium.Future work could fine-tune plant and root-associated microorganisms to growth and nutrient dynamics.
Keywords: Brachypodium; Herbaspirillum seropedicae; Agriculture; EcoFAB; N fixation; cereals; crop productivity; nitrogen; non-invasive phenotyping; plant-growth promoting rhizobacteria; wheat.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Arsova B, Foster KJ, Shelden MC, Bramley H, Watt M.. 2020. Dynamics in plant roots and shoots minimize stress, save energy and maintain water and nutrient uptake. New Phytologist 225, 1111–1119. - PubMed
-
- Boot RG, Mensink M.. 1990. Size and morphology of root systems of perennial grasses from contrasting habitats as affected by nitrogen supply. Plant and Soil 129, 291–299.
-
- Bundesministerium der justiz und für Verbraucherschutz. 2017. Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen (Düngeverordnung – DüV).https://www.gesetze-im-internet.de/d_v_2017/D%C3%BCV.pdf
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

