Development of an environmental contamination model to simulate the microbial bloom that occurs in commercial hatch cabinets
- PMID: 35512499
- PMCID: PMC9079238
- DOI: 10.1016/j.psj.2022.101890
Development of an environmental contamination model to simulate the microbial bloom that occurs in commercial hatch cabinets
Abstract
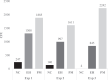
Microbial blooms that emerge in commercial hatch cabinets consist of apathogenic and pathogenic microorganisms, including Escherichia coli, Enterococcus faecalis, and Aspergillus fumigatus. Objectives of the present study included the development of a multipathogen contamination model to mimic commercial conditions and optimization of sampling methods to quantify bacterial or fungal presence within the hatch cabinet. The pathogen challenge mix (PM) was recreated from select bacterial or fungal isolates recovered from an egg homogenate (EH) derived from the contents of infertile eggs and late embryonic mortalities. Isolates selected for PM included Enterococcus faecalis (∼108 CFU/egg), Staphylococcus aureus (∼107 CFU/egg), Staphylococcus chromogenes (∼107 CFU/egg), Aspergillus fumigatus (∼106 spores/egg), and 2 Escherichia coli (∼108 CFU/egg) isolates. Challenge (100 μL of PM or EH) was administered using a sterile loop to a 28 mm area on the blunt end of the eggshell at day 19 of embryogenesis (DOE). In 3 experiments, microbiological data were collected from environmental hatcher samples (open-agar plate method), fluff samples, postmortem whole-body chick rinse samples, and gastrointestinal tract (GIT) samples to evaluate select bacteria and fungi circulating within the hatch cabinet and colonization of GIT. Cumulative bacterial and fungal recovery from the PM hatching environment from DOE20 to hatch was higher than the nonchallenged group (NC) and EH group at ∼860 and ∼1,730 CFU, respectively. Bacterial recovery from GIT, fluff, and chick rinse samples were similar for the PM and EH group in Exp. 1. However, Aspergillus fumigatus recovery from fluff and chick rinse samples for the PM group was significantly (P < 0.001) higher than the NC and EH group. In Exp. 2 and 3, PM challenge significantly (P < 0.05) increased Gram-negative bacterial recovery from the GIT, fluff and chick rinse samples compared to both the NC and EH group. These data suggest this innovative multispecies environmental contamination model using PM could be utilized to evaluate strategies to mitigate microbial contamination in commercial hatch cabinets in a laboratory setting.
Keywords: broiler; challenge; hatchery; model; pathogen.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES The authors have no conflicts of interest to report.
Figures
References
-
- Bailey J.S., Cox N.A., Berrang M.E. Hatchery-acquired salmonellae in broiler chicks. Poult. Sci. 1994;73:1153–1157. - PubMed
-
- Berrang M., Cox N., Bailey J. Measuring air-borne microbial contamination of broiler hatching cabinets. J. App. Poult. Res. 1995;4:83–87.
-
- Berrang M.E., Cox N.A., Frank J.F., Buhr R.J. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. J. App. Poult. Res. 1999;8:499–504.
-
- Board R.G., Halls N.A. The cuticle: a barrier to liquid and particle penetration of the shell of the hen's egg. Br. Poult. Sci. 1973;14:69–97. - PubMed
-
- Byrd J., DeLoach J., Corrier D., Nisbet D., Stanker L. Evaluation of Salmonella serotype distributions from commercial broiler hatcheries and grower houses. Avian. Dis. 1999;43:39–47. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

