X-ray fluorescence microscopy methods for biological tissues
- PMID: 35512669
- PMCID: PMC9226457
- DOI: 10.1093/mtomcs/mfac032
X-ray fluorescence microscopy methods for biological tissues
Abstract
Synchrotron-based X-ray fluorescence microscopy is a flexible tool for identifying the distribution of trace elements in biological specimens across a broad range of sample sizes. The technique is not particularly limited by sample type and can be performed on ancient fossils, fixed or fresh tissue specimens, and in some cases even live tissue and live cells can be studied. The technique can also be expanded to provide chemical specificity to elemental maps, either at individual points of interest in a map or across a large field of view. While virtually any sample type can be characterized with X-ray fluorescence microscopy, common biological sample preparation methods (often borrowed from other fields, such as histology) can lead to unforeseen pitfalls, resulting in altered element distributions and concentrations. A general overview of sample preparation and data-acquisition methods for X-ray fluorescence microscopy is presented, along with outlining the general approach for applying this technique to a new field of investigation for prospective new users. Considerations for improving data acquisition and quality are reviewed as well as the effects of sample preparation, with a particular focus on soft tissues. The effects of common sample pretreatment steps as well as the underlying factors that govern which, and to what extent, specific elements are likely to be altered are reviewed along with common artifacts observed in X-ray fluorescence microscopy data.
Keywords: X-ray fluorescence microscopy; XFI; XFM; biological samples; chemical speciation; imaging; mapping.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

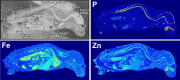






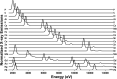


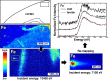




Similar articles
-
Sample Preparation for X-Ray Fluorescence Microscopy of Iron Distribution in Biological Specimen.Methods Mol Biol. 2024;2839:43-52. doi: 10.1007/978-1-0716-4043-2_3. Methods Mol Biol. 2024. PMID: 39008247
-
Visualizing Metal Distribution in Plants Using Synchrotron X-Ray Fluorescence Microscopy Techniques.Methods Mol Biol. 2023;2665:177-189. doi: 10.1007/978-1-0716-3183-6_14. Methods Mol Biol. 2023. PMID: 37166601
-
Synchrotron XFM tomography for elucidating metals and metalloids in hyperaccumulator plants.Metallomics. 2022 Nov 23;14(11):mfac069. doi: 10.1093/mtomcs/mfac069. Metallomics. 2022. PMID: 36099903 Free PMC article.
-
Advances in visualization of copper in mammalian systems using X-ray fluorescence microscopy.Curr Opin Chem Biol. 2020 Apr;55:19-25. doi: 10.1016/j.cbpa.2019.12.002. Epub 2020 Jan 3. Curr Opin Chem Biol. 2020. PMID: 31911338 Free PMC article. Review.
-
X-ray fluorescence microprobe imaging in biology and medicine.J Cell Biochem. 2006 Dec 15;99(6):1489-502. doi: 10.1002/jcb.21047. J Cell Biochem. 2006. PMID: 17006954 Review.
Cited by
-
Multimodal imaging of hemorrhagic transformation biomarkers in an ischemic stroke model.Metallomics. 2022 Apr 30;14(4):mfac007. doi: 10.1093/mtomcs/mfac007. Metallomics. 2022. PMID: 35254441 Free PMC article.
-
Correlative single-cell hard X-ray computed tomography and X-ray fluorescence imaging.Commun Biol. 2024 Mar 7;7(1):280. doi: 10.1038/s42003-024-05950-y. Commun Biol. 2024. PMID: 38448784 Free PMC article.
-
X-ray fluorescence mapping of brain tissue reveals the profound extent of trace element dysregulation in stroke pathophysiology.Metallomics. 2024 Dec 2;16(12):mfae054. doi: 10.1093/mtomcs/mfae054. Metallomics. 2024. PMID: 39547935 Free PMC article.
-
Recent advances in the application of ionomics in metabolic diseases.Front Nutr. 2023 Jan 16;9:1111933. doi: 10.3389/fnut.2022.1111933. eCollection 2022. Front Nutr. 2023. PMID: 36726817 Free PMC article. Review.
-
Intestinal Effects of Brewers' Spent Grain Extract In Ovo (Gallus gallus)-A Pilot Study.Animals (Basel). 2025 Jan 22;15(3):303. doi: 10.3390/ani15030303. Animals (Basel). 2025. PMID: 39943073 Free PMC article.
References
-
- Finney L., Mandava S., Ursos L., Zhang W., Rodi D., Vogt S., Legnini D., Maser J., Ikpatt F., Olopade O. I., Glesne D., X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis, Proc. Natl. Acad. Sci., 2007, 104, 2247–2252. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

