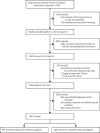
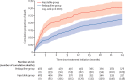
Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study
- PMID: 35512718
- PMCID: PMC9217754
- DOI: 10.1016/S1473-3099(21)00811-2
Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study
Abstract
Background: There is a need for short and safe all-oral treatment of rifampicin-resistant tuberculosis. We compared outcomes up to 24 months after treatment initiation for patients with rifampicin-resistant tuberculosis in South Africa treated with a short, all-oral bedaquiline-containing regimen (bedaquiline group), or a short, injectable-containing regimen (injectable group).
Methods: Patients with rifampicin-resistant tuberculosis, aged 18 years or older, eligible for a short regimen starting treatment between Jan 1 and Dec 31, 2017, with a bedaquiline-containing or WHO recommended injectable-containing treatment regimen of 9-12 months, registered in the drug-resistant tuberculosis database (EDRWeb), and with known age, sex, HIV status, and national identification number were eligible for study inclusion; patients receiving linezolid, carbapenems, terizidone or cycloserine, delamanid, or para-aminosalicylic acid were excluded. Bedaquiline was given at a dose of 400 mg once daily for two weeks followed by 200 mg three times a week for 22 weeks. To compare regimens, patients were exactly matched on HIV and ART status, previous tuberculosis treatment history, and baseline acid-fast bacilli smear and culture result, while propensity score matched on age, sex, province of treatment, and isoniazid-susceptibility status. We did binomial linear regression to estimate adjusted risk differences (aRD) and 95% CIs for 24-month outcomes, which included: treatment success (ie, cure or treatment completion without evidence of recurrence) versus all other outcomes, survival versus death, disease free survival versus survival with treatment failure or recurrence, and loss to follow-up versus all other outcomes.
Findings: Overall, 1387 (14%) of 10152 patients with rifampicin-resistant tuberculosis treated during 2017 met inclusion criteria; 688 in the bedaquiline group and 699 in the injectable group. Four patients (1%) had treatment failure or recurrence, 44 (6%) were lost to follow-up, and 162 (24%) died in the bedaquiline group, compared with 17 (2%), 87 (12%), and 199 (28%), respectively, in the injectable group. In adjusted analyses, treatment success was 14% (95% CI 8-20) higher in the bedaquiline group than in the injectable group (70% vs 57%); loss to follow-up was 4% (1-8) lower in the bedaquiline group (6% vs 12%); and disease-free survival was 2% (0-5) higher in the bedaquiline group (99% vs 97%). The bedaquiline group had 8% (4-11) lower risk of mortality during treatment (17·0% vs 22·4%), but there was no difference in mortality post-treatment.
Interpretation: Patients in the bedaquiline group experienced significantly higher rates of treatment success at 24 months. This finding supports the use of short bedaquiline-containing regimens in eligible patients.
Funding: WHO Global TB Programme.
Translation: For the French translation of the abstract see Supplementary Materials section.
This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Being heard on all-oral therapy for resistant tuberculosis.Lancet Infect Dis. 2022 Jul;22(7):923-924. doi: 10.1016/S1473-3099(22)00027-5. Epub 2022 May 2. Lancet Infect Dis. 2022. PMID: 35512717 No abstract available.
References
-
- WHO Global tuberculosis report 2020. 2020. https://www.who.int/publications/i/item/9789240013131
-
- Conradie F, Meintjes G, Hughes J, et al. Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. South African Med J. 2014;104:164–166. - PubMed
-
- WHO WHO treatment guidelines for drug-resistant tuberculosis. 2016. https://www.who.int/publications/i/item/9789241549639 - PubMed
-
- Ndjeka N, Schnippel K, Master I, et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J. 2018;52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous