Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via β Cys93
- PMID: 35512801
- PMCID: PMC10389762
- DOI: 10.1124/jpet.122.001194
Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via β Cys93
Abstract
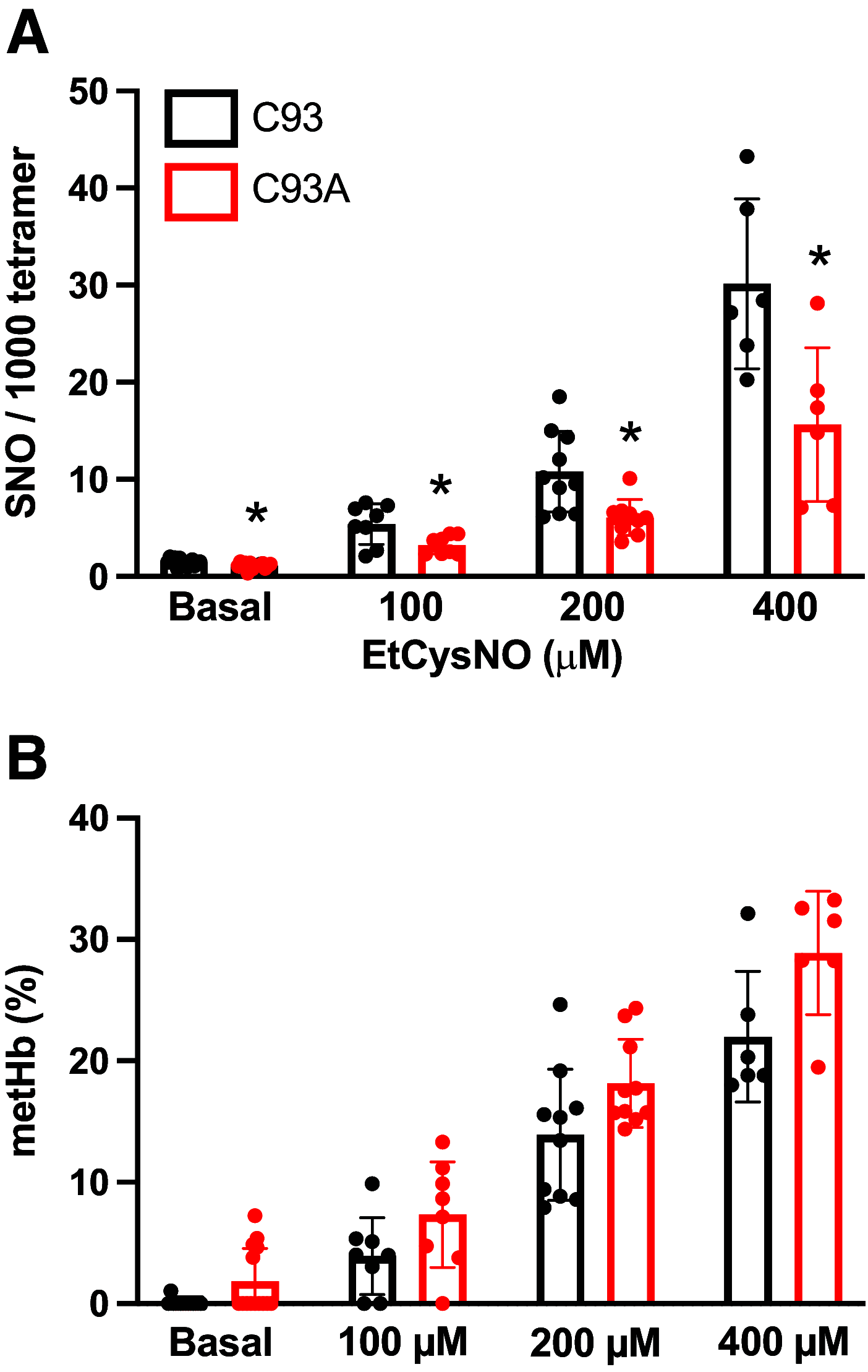
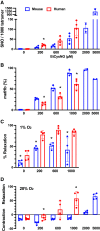
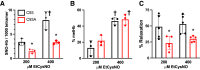
Classic physiology links tissue hypoxia to oxygen delivery through control of microvascular blood flow (autoregulation of blood flow). Hemoglobin (Hb) serves both as the source of oxygen and the mediator of microvascular blood flow through its ability to release vasodilatory S-nitrosothiol (SNO) in proportion to degree of hypoxia. β-globin Cys93Ala (βCys93Ala) mutant mice deficient in S-nitrosohemoglobin (SNO-Hb) show profound deficits in microvascular blood flow and tissue oxygenation that recapitulate microcirculatory dysfunction in multiple clinical conditions. However, the means to replete SNO in mouse red blood cells (RBCs) to restore RBC function is not known. In particular, although methods have been developed to selectively S-nitrosylate βCys93 in human Hb and intact human RBCs, conditions have not been optimized for mouse RBCs that are used experimentally. Here we show that loading SNO onto Hb in mouse RBC lysates can be achieved with high stoichiometry and β-globin selectivity. However, S-nitrosylation of Hb within intact mouse RBCs is ineffective under conditions that work well with human RBCs, and levels of metHb are prohibitively high. We developed an optimized method that loads SNO in mouse RBCs to maintain vasodilation under hypoxia and shows that loss of SNO loading in βCys93Ala mutant RBCs results in reduced vasodilation. We also demonstrate that differences in SNO/met/nitrosyl Hb stoichiometry can account for differences in RBC function among studies. RBCs loaded with quasi-physiologic amounts of SNO-Hb will produce vasodilation proportionate to hypoxia, whereas RBCs loaded with higher amounts lose allosteric regulation, thus inducing vasodilation at both high and low oxygen level. SIGNIFICANCE STATEMENT: Red blood cells from mice exhibit poor hemoglobin S-nitrosylation under conditions used for human RBCs, frustrating tests of vasodilatory activity. Using an optimized S-nitrosylation protocol, mouse RBCs exhibit hypoxic vasodilation that is significantly reduced in hemoglobin βCys93Ala mutant RBCs that cannot carry S-nitrosothiol allosterically, providing genetic validation for the role of βCys93 in oxygen delivery.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

Similar articles
-
Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review.
-
Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology.Physiology (Bethesda). 2020 Jul 1;35(4):234-243. doi: 10.1152/physiol.00040.2019. Physiology (Bethesda). 2020. PMID: 32490751 Free PMC article. Review.
-
Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
-
Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia.Proc Natl Acad Sci U S A. 2015 May 19;112(20):6425-30. doi: 10.1073/pnas.1502285112. Epub 2015 Mar 25. Proc Natl Acad Sci U S A. 2015. PMID: 25810253 Free PMC article.
-
SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article.
Cited by
-
Control of tissue oxygenation by S-nitrosohemoglobin in human subjects.Proc Natl Acad Sci U S A. 2023 Feb 28;120(9):e2220769120. doi: 10.1073/pnas.2220769120. Epub 2023 Feb 22. Proc Natl Acad Sci U S A. 2023. PMID: 36812211 Free PMC article.
-
L-Arginine and Nitric Oxide in Vascular Regulation-Experimental Findings in the Context of Blood Donation.Nutrients. 2025 Feb 13;17(4):665. doi: 10.3390/nu17040665. Nutrients. 2025. PMID: 40004994 Free PMC article. Review.
-
Erythrocytes enhance oxygen-carrying capacity through self-regulation.Front Physiol. 2025 May 16;16:1592176. doi: 10.3389/fphys.2025.1592176. eCollection 2025. Front Physiol. 2025. PMID: 40453108 Free PMC article. Review.
References
-
- Chan NL, Rogers PH, Arnone A (1998) Crystal structure of the S-nitroso form of liganded human hemoglobin. Biochemistry 37:16459–16464. - PubMed
-
- Chua CG, Carrell RW (1974) Three cysteine residues in the α chain of rat haemoglobin (albino Rattus norvegicus): 13 (A 11), 104 (G 11) and 111 (G 18). Biochim Biophys Acta 365:328–334. - PubMed
-
- Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP (2004) Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood 104:1375–1382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

