Prediction of Carboxylesterase 1-mediated In Vivo Drug Interaction between Methylphenidate and Cannabinoids using Static and Physiologically Based Pharmacokinetic Models
- PMID: 35512806
- PMCID: PMC11022897
- DOI: 10.1124/dmd.121.000823
Prediction of Carboxylesterase 1-mediated In Vivo Drug Interaction between Methylphenidate and Cannabinoids using Static and Physiologically Based Pharmacokinetic Models
Abstract
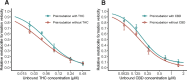
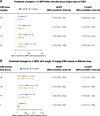
The use of cannabis products has increased substantially. Cannabis products have been perceived and investigated as potential treatments for attention-deficit/hyperactivity disorder (ADHD). Accordingly, co-administration of cannabis products and methylphenidate (MPH), a first-line medication for ADHD, is possible. Oral MPH undergoes extensive presystemic metabolism by carboxylesterase 1 (CES1), a hepatic enzyme which can be inhibited by two prominent cannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). This prompts further investigation into the likelihood of clinical interactions between MPH and these two cannabinoids through CES1 inhibition. In the present study, inhibition parameters were obtained from a human liver S9 system and then incorporated into static and physiologically-based pharmacokinetic (PBPK) models for prediction of potential clinical significance. The inhibition of MPH hydrolysis by THC and CBD was reversible, with estimated unbound inhibition constants (Ki,u) of 0.031 and 0.091 µM, respectively. The static model predicted a mild increase in MPH exposure by concurrent THC (34%) and CBD (94%) from smoking a cannabis cigarette and ingestion of prescriptive CBD, respectively. PBPK models suggested no significant interactions between single doses of MPH and CBD (2.5 - 10 mg/kg) when administered simultaneously, while a mild interaction (area under drug concentration-time curve increased by up to 55% and maximum concentration by up to 45%) is likely if multiple doses of CBD (10 mg/kg twice daily) are administered. In conclusion, the pharmacokinetic disposition of MPH can be potentially influenced by THC and CBD under certain clinical scenarios. Whether the magnitude of predicted interactions translates into clinically relevant outcomes requires verification in an appropriately designed clinical study. SIGNIFICANCE STATEMENT: This work demonstrated a potential mechanism of drug-drug interactions between methylphenidate (MPH) and two major cannabinoids (Δ9-tetrahydrocannabinol [THC] and cannabidiol [CBD]) not previously reported. We predicted a mild interaction between MPH and THC when the cannabinoid exposure occurred via cannabis smoking. Mild interactions between MPH and CBD were predicted with multiple oral administrations of CBD.
U.S. Government work not protected by U.S. copyright.
Figures




References
-
- Abbas R, Palumbo D, Walters F, Belden H, Berry SA (2016) Single-dose Pharmacokinetic Properties and Relative Bioavailability of a Novel Methylphenidate Extended-release Chewable Tablet Compared With Immediate-release Methylphenidate Chewable Tablet. Clin Ther 38:1151–1157. - PubMed
-
- Adjei A, Teuscher NS, Kupper RJ, Chang WW, Greenhill L, Newcorn JH, Connor DF, Wigal S (2014) Single-dose pharmacokinetics of methylphenidate extended-release multiple layer beads administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin(®)) in healthy adult volunteers. J Child Adolesc Psychopharmacol 24:570–578. - PMC - PubMed
-
- Aresti-Sanz J, Maho W, Pereira RR, Permentier H, El Aidy SpH-dependent spontaneous hydrolysis rather than gut bacterial metabolism reduces levels of the ADHD treatment, Methylphenidate [biRxiv website]. July 2020. Available at: https://www.biorxiv.org/content/10.1101/2020.07.06.189191v1.article-info. Accessed January 27, 2021. - DOI
-
- Azofeifa A, Mattson ME, Grant A (2016) Monitoring Marijuana Use in the United States: Challenges in an Evolving Environment. JAMA 316:1765–1766. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

