Analyzing efficacy, stability, and safety of AAV-mediated optogenetic hearing restoration in mice
- PMID: 35512833
- PMCID: PMC9258265
- DOI: 10.26508/lsa.202101338
Analyzing efficacy, stability, and safety of AAV-mediated optogenetic hearing restoration in mice
Abstract
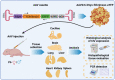
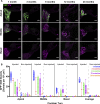
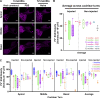
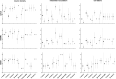
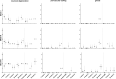
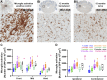
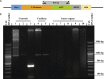
AAV-mediated optogenetic neural stimulation has become a clinical approach for restoring function in sensory disorders and feasibility for hearing restoration has been indicated in rodents. Nonetheless, long-term stability and safety of AAV-mediated channelrhodopsin (ChR) expression in spiral ganglion neurons (SGNs) remained to be addressed. Here, we used longitudinal studies on mice subjected to early postnatal administration of AAV2/6 carrying fast gating ChR f-Chrimson under the control of the human synapsin promoter unilaterally to the cochlea. f-Chrimson expression in SGNs in both ears and the brain was probed in animals aged 1 mo to 2 yr. f-Chrimson was observed in SGNs at all ages indicating longevity of ChR-expression. SGN numbers in the AAV-injected cochleae declined with age faster than in controls. Investigations were extended to the brain in which viral transduction was observed across the organ at varying degrees irrespective of age without observing viral spread-related pathologies. No viral DNA or virus-related histopathological findings in visceral organs were encountered. In summary, our study demonstrates life-long (24 mo in mice) expression of f-Chrimson in SGNs upon single AAV-dosing of the cochlea.
© 2022 Bali et al.
Conflict of interest statement
T Moser is co-founder of OptoGenTech company.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
