Longitudinal Aspects of VITT
- PMID: 35512899
- PMCID: PMC8898788
- DOI: 10.1053/j.seminhematol.2022.03.001
Longitudinal Aspects of VITT
Abstract
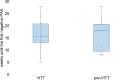
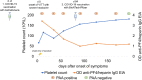
In hundreds of patients worldwide, vaccination against COVID-19 with adenovirus vector vaccines (ChAdOx1 nCoV-19; Ad26.COV2.S) triggered platelet-activating anti-platelet factor 4 (PF4) antibodies inducing vaccine-induced immune thrombotic thrombocytopenia (VITT). In most VITT patients, platelet-activating anti-PF4-antibodies are transient and the disorder is discrete and non-recurring. However, in some patients platelet-activating antibodies persist, associated with recurrent thrombocytopenia and sometimes with relapse of thrombosis despite therapeutic-dose anticoagulation. Anti-PF4 IgG antibodies measured by enzyme-immunoassay (EIA) are usually detectable for longer than platelet-activating antibodies in functional assays, but duration of detectability is highly assay-dependent. As more than 1 vaccination dose against COVID-19 is required to achieve sufficient protection, at least 69 VITT patients have undergone subsequent vaccination with an mRNA vaccine, with no relevant subsequent increase in anti-PF4 antibody titers, thrombocytopenia, or thrombotic complications. Also, re-exposure to adenoviral vector-based vaccines in 5 VITT patients was not associated with adverse reactions. Although data are limited, vaccination against influenza also appears to be safe. SARS-CoV-2 infection reported in 1 patient with preceding VITT did not influence anti-PF4 antibody levels. We discuss how these temporal characteristics of VITT provide insights into pathogenesis.
Keywords: COVID-19; ChAdOx1 nCoV-19; TTS; VITT; platelet factor 4; vaccination.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
-
Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT).Semin Hematol. 2022 Apr;59(2):97-107. doi: 10.1053/j.seminhematol.2022.02.004. Epub 2022 Feb 23. Semin Hematol. 2022. PMID: 35512907 Free PMC article.
-
Biophysical studies do not reveal direct interactions between human PF4 and Ad26.COV2.S vaccine.J Thromb Haemost. 2024 Apr;22(4):1046-1055. doi: 10.1016/j.jtha.2023.12.020. Epub 2023 Dec 29. J Thromb Haemost. 2024. PMID: 38159648
-
Epidemiology of VITT.Semin Hematol. 2022 Apr;59(2):72-75. doi: 10.1053/j.seminhematol.2022.02.002. Epub 2022 Feb 8. Semin Hematol. 2022. PMID: 35512903 Free PMC article.
-
Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection.J Autoimmun. 2021 Jul;121:102662. doi: 10.1016/j.jaut.2021.102662. Epub 2021 May 19. J Autoimmun. 2021. PMID: 34051613 Free PMC article. Review.
Cited by
-
Vaccine-induced immune thrombotic thrombocytopenia.Best Pract Res Clin Haematol. 2022 Sep;35(3):101381. doi: 10.1016/j.beha.2022.101381. Epub 2022 Sep 13. Best Pract Res Clin Haematol. 2022. PMID: 36494147 Free PMC article. Review.
-
Portal Vein and Mesenteric Artery Thrombosis Following the Administration of an Ad26.COV2-S Vaccine-First Case from Romania: A Case Report.Vaccines (Basel). 2022 Nov 18;10(11):1950. doi: 10.3390/vaccines10111950. Vaccines (Basel). 2022. PMID: 36423045 Free PMC article.
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
-
Risk of relapse after SARS-CoV-2 vaccine in the Milan cohort of thrombotic thrombocytopenic purpura patients.Haematologica. 2023 Nov 1;108(11):3152-3155. doi: 10.3324/haematol.2022.282478. Haematologica. 2023. PMID: 36951158 Free PMC article. No abstract available.
-
Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT)-Insights from Clinical Cases, In Vitro Studies and Murine Models.J Clin Med. 2023 Sep 22;12(19):6126. doi: 10.3390/jcm12196126. J Clin Med. 2023. PMID: 37834770 Free PMC article. Review.
References
-
- World Health Organization. Report of the independent allocation of vaccines group on the allocation of COVAX facility secured vaccines. https://www.who.int/publications/m/item/report-of-the-independent-alloca... [accessed September 09, 2021].
-
- WHO. COVID-19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... [accessed September 13, 2021].
-
- Paul-Ehrlich-Institut. Sicherheitsbericht. https://www.pei.de/SharedDocs/Downloads/DE/newsroom/dossiers/sicherheits.... [accessed July 18, 2021].
-
- Government United Kingdom. Coronavirus vaccine - weekly summary of Yellow Card reporting. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-... [accessed July 18, 2021].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

