Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT)
- PMID: 35512907
- PMCID: PMC8863951
- DOI: 10.1053/j.seminhematol.2022.02.004
Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT)
Abstract
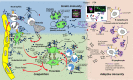
Vaccine-induced immune thrombotic thrombocytopenia (VITT; synonym, thrombosis with thrombocytopenia syndrome, is associated with high-titer immunoglobulin G antibodies directed against platelet factor 4 (PF4). These antibodies activate platelets via platelet FcγIIa receptors, with platelet activation greatly enhanced by PF4. Here we summarize the current concepts in the pathogenesis of VITT. We first address parallels between heparin-induced thrombocytopenia and VITT, and provide recent findings on binding of PF4 to adenovirus particles and non-assembled adenovirus proteins in the 2 adenovirus vector-based COVID-19 vaccines, ChAdOx1 nCoV-19 and Ad26.COV2.S. Further, we discuss the potential role of vaccine constituents such as glycosaminoglycans, EDTA, polysorbate 80, human cell-line proteins and nucleotides as potential binding partners of PF4. The immune response towards PF4 in VITT is likely triggered by a proinflammatory milieu. Human cell-line proteins, non-assembled virus proteins, and potentially EDTA may contribute to the proinflammatory state. The transient nature of the immune response towards PF4 in VITT makes it likely that-as in heparin-induced thrombocytopenia -marginal zone B cells are key for antibody production. Once high-titer anti-PF4 antibodies have been formed 5 to 20 days after vaccination, they activate platelets and granulocytes. Activated granulocytes undergo NETosis and the released DNA also forms complexes with PF4, which fuels the Fcγ receptor-dependent cell activation process, ultimately leading to massive thrombin generation. Finally, we summarize our initial observations indicating that VITT-like antibodies might also be present in rare patients with recurrent venous and arterial thrombotic complications, independent of vaccination.
Keywords: COVID-19; ChAdOx1 nCoV-19; Platelet factor 4; TTS; VITT; Vaccination.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest Dr Schönborn is the recipient of a young investigator grant of the medical faculty of the Universitätsmedizin Greifswald. Dr. Greinacher reports grants and non-financial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, Instrumentation Laboratory, nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. Dr. Thiele reports personal fees from Bristol Myers Squibb, Bayer, Daichii Sankyo, Pfizer, Novo Nordisk, Chugai Pharma, and Novartis, all of which are outside of the submitted manuscript. None of the other authors has to declare a conflict of interest.
Figures


Similar articles
-
Potential mechanisms of vaccine-induced thrombosis.Eur J Intern Med. 2022 Nov;105:1-7. doi: 10.1016/j.ejim.2022.08.002. Epub 2022 Aug 8. Eur J Intern Med. 2022. PMID: 35953336 Free PMC article. Review.
-
Longitudinal Aspects of VITT.Semin Hematol. 2022 Apr;59(2):108-114. doi: 10.1053/j.seminhematol.2022.03.001. Epub 2022 Mar 7. Semin Hematol. 2022. PMID: 35512899 Free PMC article.
-
Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia.Blood. 2021 Dec 2;138(22):2256-2268. doi: 10.1182/blood.2021013231. Blood. 2021. PMID: 34587242 Free PMC article.
-
Biophysical studies do not reveal direct interactions between human PF4 and Ad26.COV2.S vaccine.J Thromb Haemost. 2024 Apr;22(4):1046-1055. doi: 10.1016/j.jtha.2023.12.020. Epub 2023 Dec 29. J Thromb Haemost. 2024. PMID: 38159648
-
Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection.J Autoimmun. 2021 Jul;121:102662. doi: 10.1016/j.jaut.2021.102662. Epub 2021 May 19. J Autoimmun. 2021. PMID: 34051613 Free PMC article. Review.
Cited by
-
'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA.Biomedicines. 2023 Aug 17;11(8):2287. doi: 10.3390/biomedicines11082287. Biomedicines. 2023. PMID: 37626783 Free PMC article. Review.
-
Incidence and management of the main serious adverse events reported after COVID-19 vaccination.Pharmacol Res Perspect. 2024 Jun;12(3):e1224. doi: 10.1002/prp2.1224. Pharmacol Res Perspect. 2024. PMID: 38864106 Free PMC article. Review.
-
Lipid signatures of immunothrombosis: insights from VITT.Res Pract Thromb Haemost. 2025 Mar 6;9(2):102725. doi: 10.1016/j.rpth.2025.102725. eCollection 2025 Feb. Res Pract Thromb Haemost. 2025. PMID: 40224271 Free PMC article. No abstract available.
-
Case Report: Anti-platelet factor 4 -mediated immunothrombosis in a patient with ANCA vasculitis - a shared mechanism of NETosis.Front Immunol. 2025 Apr 10;16:1567999. doi: 10.3389/fimmu.2025.1567999. eCollection 2025. Front Immunol. 2025. PMID: 40276517 Free PMC article.
-
COVID-19 Adenoviral Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT), COVID-19-Related Thrombosis, and the Thrombotic Thrombocytopenic Syndromes.Hematol Rep. 2022 Dec 1;14(4):358-372. doi: 10.3390/hematolrep14040050. Hematol Rep. 2022. PMID: 36547234 Free PMC article. Review.
References
-
- European Medicines Agency (EMA) Vaxzevria (previously covid-19 vaccine astra zeneca): Epar - product information. 2021 [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-c... accessed February 28, 2022.
-
- European Medicines Agency & Healthcare products Regulatory Agency- Public assessment report authorisation for temporary supply - covid-19 vaccine astrazeneca, solution for injection in multidose container covid-19 vaccine (chadox1-s [recombinant]). 2021 [Available from: https://www.ema.europa.eu/en/documents/assessment-report/vaxzevria-previ... accessed February 28, 2022.
-
- European Medicines Agency (EMA) COVID-19 Vaccine Janssen (COVID-19 vaccine (Ad26.COV2-S [recombinant])) 2021 [Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen accessed February 28, 2022.
-
- Krauel K, Potschke C, Weber C, et al. Platelet factor 4 binds to bacteria-inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. - PubMed
-
- Palankar R, Kohler TP, Krauel K, et al. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and FcgammaRIIA. J Thromb Haemost. 2018;16:1187–1197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

