The role of Iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice
- PMID: 35513057
- PMCID: PMC9843742
- DOI: 10.1016/j.taap.2022.116043
The role of Iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice
Abstract
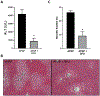
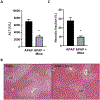
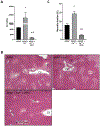
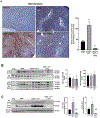
Acetaminophen (APAP) hepatotoxicity, a leading cause of acute liver failure in western countries, is characterized by mitochondrial superoxide and peroxynitrite formation. However, the role of iron, especially as facilitator of lipid peroxidation (LPO), has been controversial. Our aim was to determine the mechanism by which iron promotes cell death in this context. Fasted male C57BL/6J mice were treated with the iron chelator deferoxamine, minocycline (inhibitor of the mitochondrial calcium uniporter) or vehicle 1 h before 300 mg/kg APAP. Deferoxamine and minocycline significantly attenuated APAP-induced elevations in serum alanine amino transferase levels and hepatic necrosis at 6 h. This protection correlated with reduced 3-nitro-tyrosine protein adducts; LPO (malondialdehyde, 4-hydroxynonenal) was not detected. Activation of c-jun N-terminal kinase (JNK) was not affected but mitochondrial release of intermembrane proteins was reduced suggesting that the effect of iron was at the level of mitochondria. Co-treatment of APAP with FeSO4 exacerbated liver injury and protein nitration and triggered significant LPO; all effects were reversed by deferoxamine. Thus, after APAP overdose, iron imported into mitochondria facilitates protein nitration by peroxynitrite triggering mitochondrial dysfunction and cell death. Under these conditions, endogenous defense mechanisms largely prevent LPO. However, after iron overload, protein nitration and LPO contribute to APAP hepatotoxicity.
Keywords: Acetaminophen Hepatotoxicity; Deferoxamine; Ferrous iron; Lipid Peroxidation; Minocycline; Peroxynitrite.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures




References
-
- Audimoolam VK, Wendon J, Bernal W, Heaton N, O’Grady J, Auzinger G, 2011. Iron and acetaminophen a fatal combination? Transpl. Int 24, e85–e88. - PubMed
-
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H, 2008. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther 324, 8–14. - PubMed
-
- Bernal W, Wendon J, 2013. Acute liver failure. N. Engl. J. Med 369, 2525–2534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

