Hippocampus-sensitive and striatum-sensitive learning one month after morphine or cocaine exposure in male rats
- PMID: 35513118
- PMCID: PMC9796089
- DOI: 10.1016/j.pbb.2022.173392
Hippocampus-sensitive and striatum-sensitive learning one month after morphine or cocaine exposure in male rats
Abstract
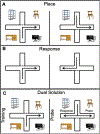
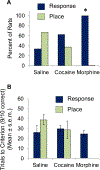
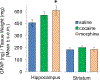
These experiments examined whether morphine and cocaine alter the balance between hippocampal and striatal memory systems measured long after drug exposure. Male rats received injections of morphine (5 mg/kg), cocaine (20 mg/kg), or saline for five consecutive days. One month later, rats were trained to find food on a hippocampus-sensitive place task or a striatum-sensitive response task. Relative to saline controls, morphine-treated rats exhibited impaired place learning but enhanced response learning; prior cocaine exposure did not significantly alter learning on either task. Another set of rats was trained on a dual-solution T-maze that can be solved with either place or response strategies. While a majority (67%) of control rats used place solutions, morphine treatment one month prior resulted in the exclusive use of response solutions (100%). Prior cocaine treatment did not significantly alter strategy selection. Molecular markers related to learning and drug abuse were measured in the hippocampus and striatum one month after drug exposure in behaviorally untested rats. Protein levels of glial-fibrillary acidic protein (GFAP), an intermediate filament specific to astrocytes, increased significantly in the hippocampus after morphine exposure, but not after cocaine exposure. Exposure to morphine or cocaine did not significantly change levels of brain-derived neurotrophic factor (BDNF) or a downstream target of BDNF signaling, glycogen synthase kinase 3β (GSK3β), in the hippocampus or striatum. Thus, exposure to morphine resulted in a long-lasting shift from hippocampal toward striatal dominance during learning, an effect that may be associated with lasting alterations in hippocampal astrocytes. Cocaine produced changes in the same direction, suggesting that use of a higher dose or longer duration of exposure might produce effects comparable to those seen with morphine.
Keywords: Astrocytes; BDNF; Decision-making; GFAP; GSK3β; Learning.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures


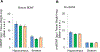

Similar articles
-
Glucose injections into the dorsal hippocampus or dorsolateral striatum of rats prior to T-maze training: modulation of learning rates and strategy selection.Learn Mem. 2005 Jul-Aug;12(4):367-74. doi: 10.1101/lm.88205. Epub 2005 Jul 18. Learn Mem. 2005. PMID: 16027177 Free PMC article.
-
BDNF and p-GSK3β in the hippocampus mediate the impairment of delay-based decision making in morphine-dependent rats.Neuroreport. 2020 Dec 9;31(17):1208-1214. doi: 10.1097/WNR.0000000000001535. Neuroreport. 2020. PMID: 33075004
-
Hippocampal BDNF regulates a shift from flexible, goal-directed to habit memory system function following cocaine abstinence.Hippocampus. 2019 Nov;29(11):1101-1113. doi: 10.1002/hipo.23127. Epub 2019 Jun 17. Hippocampus. 2019. Retraction in: Hippocampus. 2020 Dec;30(12):1327. doi: 10.1002/hipo.23277. PMID: 31206907 Free PMC article. Retracted.
-
Effects of environmental enrichment on behavioral deficits and alterations in hippocampal BDNF induced by prenatal exposure to morphine in juvenile rats.Neuroscience. 2015 Oct 1;305:372-83. doi: 10.1016/j.neuroscience.2015.08.015. Epub 2015 Aug 10. Neuroscience. 2015. PMID: 26272536
-
Deleterious effects of prenatal exposure to morphine on the spatial learning and hippocampal BDNF and long-term potentiation in juvenile rats: Beneficial influences of postnatal treadmill exercise and enriched environment.Neurobiol Learn Mem. 2018 Jan;147:54-64. doi: 10.1016/j.nlm.2017.11.013. Epub 2017 Nov 23. Neurobiol Learn Mem. 2018. PMID: 29175674
References
-
- Andero R, Choi DC, Ressler KJ, 2014. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog. Mol. Biol. Transl. Sci. 122, 169–192, 10.1016. B978-0-12-420170-5.00006-4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

