Nanofiber capsules for minimally invasive sampling of biological specimens from gastrointestinal tract
- PMID: 35513306
- PMCID: PMC9371944
- DOI: 10.1016/j.actbio.2022.04.045
Nanofiber capsules for minimally invasive sampling of biological specimens from gastrointestinal tract
Abstract
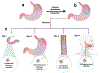
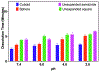
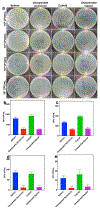
Accurate and rapid point-of-care tissue and microbiome sampling is critical for early detection of cancers and infectious diseases and often result in effective early intervention and prevention of disease spread. In particular, the low prevalence of Barrett's and gastric premalignancy in the Western world makes population-based endoscopic screening unfeasible and cost-ineffective. Herein, we report a method that may be useful for prescreening the general population in a minimally invasive way using a swallowable, re-expandable, ultra-absorbable, and retrievable nanofiber cuboid and sphere produced by electrospinning, gas-foaming, coating, and crosslinking. The water absorption capacity of the cuboid- and sphere-shaped nanofiber objects is shown ∼6000% and ∼2000% of their dry mass. In contrast, unexpanded semicircular and square nanofiber membranes showed <500% of their dry mass. Moreover, the swallowable sphere and cuboid were able to collect and release more bacteria, viruses, and cells/tissues from solutions as compared with unexpanded scaffolds. In addition to that, an expanded sphere shows higher cell collection capacity from the esophagus inner wall as compared with the unexpanded nanofiber membrane. Taken together, the nanofiber capsules developed in this study could provide a minimally invasive method of collecting biological samples from the duodenal, gastric, esophagus, and oropharyngeal sites, potentially leading to timely and accurate diagnosis of many diseases. STATEMENT OF SIGNIFICANCE: Recently, minimally invasive technologies have gained much attention in tissue engineering and disease diagnosis. In this study, we engineered a swallowable and retrievable electrospun nanofiber capsule serving as collection device to collect specimens from internal organs in a minimally invasive manner. The sample collection device could be an alternative endoscopy to collect the samples from internal organs like jejunum, stomach, esophagus, and oropharynx without any sedation. The newly engineered nanofiber capsule could be used to collect, bacteria, virus, fluids, and cells from the abovementioned internal organs. In addition, the biocompatible and biodegradable nanofiber capsule on a string could exhibit a great sample collection capacity for the primary screening of Barret Esophagus, acid reflux, SARS-COVID-19, Helicobacter pylori, and gastric cancer.
Keywords: Barret esophagus; Gastrointestinal tract; Nanofiber capsule; SARS-CoV-2; Sample collection.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures



Similar articles
-
Ultra-absorptive Nanofiber Swabs for Improved Collection and Test Sensitivity of SARS-CoV-2 and other Biological Specimens.Nano Lett. 2021 Feb 10;21(3):1508-1516. doi: 10.1021/acs.nanolett.0c04956. Epub 2021 Jan 27. Nano Lett. 2021. PMID: 33501831
-
Feasibility and safety of string, wireless capsule endoscopy in the diagnosis of Barrett's esophagus.Gastrointest Endosc. 2005 May;61(6):741-6. doi: 10.1016/s0016-5107(05)00322-6. Gastrointest Endosc. 2005. PMID: 15855985
-
Bacterial Composition of the Human Upper Gastrointestinal Tract Microbiome Is Dynamic and Associated with Genomic Instability in a Barrett's Esophagus Cohort.PLoS One. 2015 Jun 15;10(6):e0129055. doi: 10.1371/journal.pone.0129055. eCollection 2015. PLoS One. 2015. PMID: 26076489 Free PMC article.
-
Innovations in Screening Tools for Barrett's Esophagus and Esophageal Adenocarcinoma.Curr Gastroenterol Rep. 2021 Oct 15;23(12):22. doi: 10.1007/s11894-021-00821-6. Curr Gastroenterol Rep. 2021. PMID: 34654955 Review.
-
The diagnosis and management of Barrett's esophagus.Adv Surg. 1999;33:29-68. Adv Surg. 1999. PMID: 10572561 Review.
Cited by
-
New dimensions of electrospun nanofiber material designs for biotechnological uses.Trends Biotechnol. 2024 May;42(5):631-647. doi: 10.1016/j.tibtech.2023.11.008. Epub 2023 Dec 28. Trends Biotechnol. 2024. PMID: 38158307 Free PMC article. Review.
-
Improvement in probiotic intestinal survival by electrospun milk fat globule membrane-pullulan nanofibers: Fabrication and structural characterization.Food Chem X. 2024 Aug 22;23:101756. doi: 10.1016/j.fochx.2024.101756. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39295963 Free PMC article.
-
Mechanically resilient hybrid aerogels containing fibers of dual-scale sizes and knotty networks for tissue regeneration.Nat Commun. 2024 Feb 5;15(1):1080. doi: 10.1038/s41467-024-45458-x. Nat Commun. 2024. PMID: 38316777 Free PMC article.
-
Recent advances in the medical applications of hemostatic materials.Theranostics. 2023 Jan 1;13(1):161-196. doi: 10.7150/thno.79639. eCollection 2023. Theranostics. 2023. PMID: 36593953 Free PMC article. Review.
-
Sampling strategies for digestive system flora studies: current research and perspectives.PeerJ. 2025 Aug 13;13:e19810. doi: 10.7717/peerj.19810. eCollection 2025. PeerJ. 2025. PMID: 40821994 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

