HPLC-Based Automated Synthesis of Glycans in Solution
- PMID: 35513346
- PMCID: PMC9403992
- DOI: 10.1002/chem.202201180
HPLC-Based Automated Synthesis of Glycans in Solution
Abstract
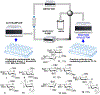
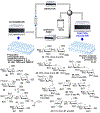
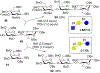
As the 21st century unfolds with rapid changes, new challenges in research and development emerge. These new challenges prompted us to repurpose our HPLC-A platform that was previously used in solid phase glycan synthesis to a solution phase batch synthesis described herein. The modular character of HPLC allows for implementing new attachments. To enable sequential synthesis of multiple oligosaccharides with the single press of a button, we supplemented our system with a four-way split valve and an automated fraction collector. This enabled the operator to load all reagents and all reactants in the autosampler, press the button to start the repetitive automation sequence, leave the lab, and upon return find products of multiple reactions ready for purification, analysis, and subsequent application.
Keywords: automation; glycans; glycosylation; oligosaccharides; synthesis.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures

Similar articles
-
HPLC-Based Automated Synthesis and Purification of Carbohydrates.Chemistry. 2024 Aug 1;30(43):e202401214. doi: 10.1002/chem.202401214. Epub 2024 May 29. Chemistry. 2024. PMID: 38684455 Free PMC article.
-
Automated Glycan Assembly of Plant Cell Wall Oligosaccharides.Methods Mol Biol. 2020;2149:503-512. doi: 10.1007/978-1-0716-0621-6_28. Methods Mol Biol. 2020. PMID: 32617953
-
HPLC-Assisted Automated Oligosaccharide Synthesis: Implementation of the Autosampler as a Mode of the Reagent Delivery.J Org Chem. 2016 Oct 7;81(19):8796-8805. doi: 10.1021/acs.joc.6b01439. Epub 2016 Sep 14. J Org Chem. 2016. PMID: 27575052 Free PMC article.
-
Strategies for Automated Enzymatic Glycan Synthesis (AEGS).Biotechnol Adv. 2023 Oct;67:108208. doi: 10.1016/j.biotechadv.2023.108208. Epub 2023 Jul 10. Biotechnol Adv. 2023. PMID: 37437855 Review.
-
Toward Automated Enzymatic Synthesis of Oligosaccharides.Chem Rev. 2018 Sep 12;118(17):8151-8187. doi: 10.1021/acs.chemrev.8b00066. Epub 2018 Jul 16. Chem Rev. 2018. PMID: 30011195 Review.
Cited by
-
HPLC-Based Automated Synthesis and Purification of Carbohydrates.Chemistry. 2024 Aug 1;30(43):e202401214. doi: 10.1002/chem.202401214. Epub 2024 May 29. Chemistry. 2024. PMID: 38684455 Free PMC article.
-
Recyclable fluorous-tag assisted two-directional oligosaccharide synthesis enabled by interrupted Pummerer reaction mediated glycosylation.Chem Sci. 2022 Jun 22;13(30):8759-8765. doi: 10.1039/d2sc01700h. eCollection 2022 Aug 4. Chem Sci. 2022. PMID: 35975149 Free PMC article.
-
Expediting Glycospace Exploration: Therapeutic Glycans via Automated Synthesis.Angew Chem Int Ed Engl. 2025 Mar 24;64(13):e202422766. doi: 10.1002/anie.202422766. Epub 2025 Feb 21. Angew Chem Int Ed Engl. 2025. PMID: 39936247 Free PMC article. Review.
-
Enhanced experimental set-up for HPLC-based automated synthesis and purification of carbohydrate building blocks.Carbohydr Res. 2025 Sep;555:109554. doi: 10.1016/j.carres.2025.109554. Epub 2025 May 27. Carbohydr Res. 2025. PMID: 40480011
-
Chemical Synthesis of Human Milk Oligosaccharides: para-Lacto-N-hexaose and para-Lacto-N-neohexaose.Chemistry. 2023 Nov 16;29(64):e202302288. doi: 10.1002/chem.202302288. Epub 2023 Oct 6. Chemistry. 2023. PMID: 37639512 Free PMC article.
References
-
- Toy PH, Lam Y in Solid-phase organic synthesis, Vol. John Wiley & Sons, Inc., Hoboken, 2012.
-
- Tanaka H, Matoba N, Tsukamoto H, Takimoto H, Yamada H, Takahashi T, Synlett 2005, 824–828;
- Machida K, Hirose Y, Fuse S, Sugawara T, Takahashi T, Chem. Pharm. Bull 2010, 58, 87–93. - PubMed
-
- Jaipuri FA, Pohl NL, Org. Biomol. Chem 2008, 6, 2686–2691; - PubMed
- Tang S-L, Linz LB, Bonning BC, Pohl NLB, J. Org. Chem 2015, 80, 10482–10489; - PMC - PubMed
- Tang S-L, Pohl NLB, Org. Lett 2015, 17, 2642–2645; - PMC - PubMed
- Nagy G, Peng T, Kabotso DEK, Novotny MV, Pohl NLB, Chem. Commun 2016, 52, 13253–13256; - PMC - PubMed
- Tang S-L, Pohl NLB, Carbohydr. Res 2016, 430, 8–15. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

