Developmental mRNA m5C landscape and regulatory innovations of massive m5C modification of maternal mRNAs in animals
- PMID: 35513466
- PMCID: PMC9072368
- DOI: 10.1038/s41467-022-30210-0
Developmental mRNA m5C landscape and regulatory innovations of massive m5C modification of maternal mRNAs in animals
Abstract
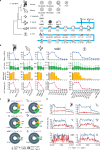
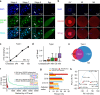
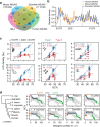
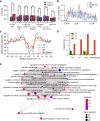
m5C is one of the longest-known RNA modifications, however, its developmental dynamics, functions, and evolution in mRNAs remain largely unknown. Here, we generate quantitative mRNA m5C maps at different stages of development in 6 vertebrate and invertebrate species and find convergent and unexpected massive methylation of maternal mRNAs mediated by NSUN2 and NSUN6. Using Drosophila as a model, we reveal that embryos lacking maternal mRNA m5C undergo cell cycle delays and fail to timely initiate maternal-to-zygotic transition, implying the functional importance of maternal mRNA m5C. From invertebrates to the lineage leading to humans, two waves of m5C regulatory innovations are observed: higher animals gain cis-directed NSUN2-mediated m5C sites at the 5' end of the mRNAs, accompanied by the emergence of more structured 5'UTR regions; humans gain thousands of trans-directed NSUN6-mediated m5C sites enriched in genes regulating the mitotic cell cycle. Collectively, our studies highlight the existence and regulatory innovations of a mechanism of early embryonic development and provide key resources for elucidating the role of mRNA m5C in biology and disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition.Nature. 2017 Feb 23;542(7642):475-478. doi: 10.1038/nature21355. Epub 2017 Feb 13. Nature. 2017. PMID: 28192787 Free PMC article.
-
Post-translational regulation of the maternal-to-zygotic transition.Cell Mol Life Sci. 2018 May;75(10):1707-1722. doi: 10.1007/s00018-018-2750-y. Epub 2018 Feb 9. Cell Mol Life Sci. 2018. PMID: 29427077 Free PMC article. Review.
-
Evolution of maternal and zygotic mRNA complements in the early Drosophila embryo.PLoS Genet. 2018 Dec 17;14(12):e1007838. doi: 10.1371/journal.pgen.1007838. eCollection 2018 Dec. PLoS Genet. 2018. PMID: 30557299 Free PMC article.
-
The conserved regulatory basis of mRNA contributions to the early Drosophila embryo differs between the maternal and zygotic genomes.PLoS Genet. 2020 Mar 30;16(3):e1008645. doi: 10.1371/journal.pgen.1008645. eCollection 2020 Mar. PLoS Genet. 2020. PMID: 32226006 Free PMC article.
-
The maternal-to-zygotic transition revisited.Development. 2019 Jun 12;146(11):dev161471. doi: 10.1242/dev.161471. Development. 2019. PMID: 31189646 Review.
Cited by
-
Base-Resolution Sequencing Methods for Whole-Transcriptome Quantification of mRNA Modifications.Acc Chem Res. 2024 Jan 2;57(1):47-58. doi: 10.1021/acs.accounts.3c00532. Epub 2023 Dec 11. Acc Chem Res. 2024. PMID: 38079380 Free PMC article.
-
PCBP1/2 and TDP43 Function as NAT10 Adaptors to Mediate mRNA ac4C Formation in Mammalian Cells.Adv Sci (Weinh). 2024 Dec;11(47):e2400133. doi: 10.1002/advs.202400133. Epub 2024 Nov 18. Adv Sci (Weinh). 2024. PMID: 39556689 Free PMC article.
-
Transcriptome-wide RNA 5-methylcytosine profiles of human iPSCs and iPSC-derived cardiomyocytes.Sci Data. 2024 Dec 18;11(1):1378. doi: 10.1038/s41597-024-04209-9. Sci Data. 2024. PMID: 39695135 Free PMC article.
-
The maternal-to-zygotic transition: reprogramming of the cytoplasm and nucleus.Nat Rev Genet. 2025 Apr;26(4):245-267. doi: 10.1038/s41576-024-00792-0. Epub 2024 Nov 25. Nat Rev Genet. 2025. PMID: 39587307 Free PMC article. Review.
-
The regulation of antiviral innate immunity through non-m6A RNA modifications.Front Immunol. 2023 Oct 17;14:1286820. doi: 10.3389/fimmu.2023.1286820. eCollection 2023. Front Immunol. 2023. PMID: 37915585 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

