Harnessing the liver to induce antigen-specific immune tolerance
- PMID: 35513495
- PMCID: PMC9256566
- DOI: 10.1007/s00281-022-00942-8
Harnessing the liver to induce antigen-specific immune tolerance
Abstract
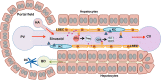
Autoimmune diseases develop when the adaptive immune system attacks the body's own antigens leading to tissue damage. At least 80 different conditions are believed to have an autoimmune aetiology, including rheumatoid arthritis, type I diabetes, multiple sclerosis or systemic lupus erythematosus. Collectively, autoimmune diseases are a leading cause of severe health impairment along with substantial socioeconomic costs. Current treatments are mostly symptomatic and non-specific, and it is typically not possible to cure these diseases. Thus, the development of more causative treatments that suppress only the pathogenic immune responses, but spare general immunity is of great biomedical interest. The liver offers considerable potential for development of such antigen-specific immunotherapies, as it has a distinct physiological capacity to induce immune tolerance. Indeed, the liver has been shown to specifically suppress autoimmune responses to organ allografts co-transplanted with the liver or to autoantigens that were transferred to the liver. Liver tolerance is established by a unique microenvironment that facilitates interactions between liver-resident antigen-presenting cells and lymphocytes passing by in the low blood flow within the hepatic sinusoids. Here, we summarise current concepts and mechanisms of liver immune tolerance, and review present approaches to harness liver tolerance for antigen-specific immunotherapy.
Keywords: Antigen presentation; Autoimmune disease; Immune tolerance; Immunotherapy; Nanomedicine; Scavenger cells.
© 2022. The Author(s).
Conflict of interest statement
This work was supported by the Deutsche Forschungsgemeinschaft—DFG (SFB841), the German Federal Ministry of Education and Research (16GW0051, 13XP5079C) and the European Regional Development Fund (OpToPas). CG has no relevant financial or non-financial interests to disclose. AC and JH are inventors of a patent related to this work (EP 2780036 (B1)). JH has consulted Topas Therapeutics GmbH without compensation.
Figures

References
-
- NIH Autoimmune Diseases Coordinating Committee (2005) Progress in autoimmune diseases research. https://www.niaid.nih.gov/sites/default/files/adccfinal.pdf. Accessed 13 January 2022
-
- Culina S, Lalanne AI, Afonso G, Cerosaletti K, Pinto S, Sebastiani G, Kuranda K, Nigi L, Eugster A, Østerbye T, Maugein A, McLaren JE, Ladell K, Larger E, Beressi JP, Lissina A, Appay V, Davidson HW, Buus S, Price DA, Kuhn M, Bonifacio E, Battaglia M, Caillat-Zucman S, Dotta F, Scharfmann R, Kyewski B, Mallone R, ImMaDiab Study Group Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol. 2018;3:eaao4013. doi: 10.1126/sciimmunol.aao4013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

