Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke
- PMID: 35513682
- PMCID: PMC9554175
- DOI: 10.1007/s12264-022-00859-0
Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke
Abstract
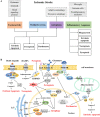
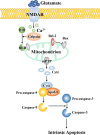
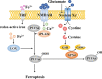
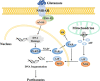
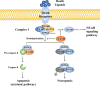
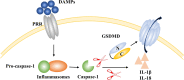
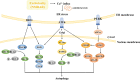
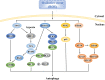
Ischemic stroke caused by intracranial vascular occlusion has become increasingly prevalent with considerable mortality and disability, which gravely burdens the global economy. Current relatively effective clinical treatments are limited to intravenous alteplase and thrombectomy. Even so, patients still benefit little due to the short therapeutic window and the risk of ischemia/reperfusion injury. It is therefore urgent to figure out the neuronal death mechanisms following ischemic stroke in order to develop new neuroprotective strategies. Regarding the pathogenesis, multiple pathological events trigger the activation of cell death pathways. Particular attention should be devoted to excitotoxicity, oxidative stress, and inflammatory responses. Thus, in this article, we first review the principal mechanisms underlying neuronal death mediated by these significant events, such as intrinsic and extrinsic apoptosis, ferroptosis, parthanatos, pyroptosis, necroptosis, and autophagic cell death. Then, we further discuss the possibility of interventions targeting these pathological events and summarize the present pharmacological achievements.
Keywords: Ischemic stroke; Mechanisms; Neuronal death; Therapeutic strategy.
© 2022. The Author(s).
Conflict of interest statement
All authors claim that there are no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

